Preface
On a beautiful NY evening, an Indian-origin chemical engineer suddenly has an ‘urge’ to go serve Mother India. He, along with his few pals, came to join a major Indian pharma company. And in the name of serving, they ‘exposed’ what some claimed was some documentation and trial error, bagging millions of dollars in whistle-blower clause from the US Food & Drug Administration (FDA). And then, they happily return to the US. The hero and his pals.
Did we mention that his heart was so much rooting for India that he had discarded Indian citizenship and taken US citizenship just before the urge to serve his ‘motherland’. Did we mention that the Indian company in question was posing a major challenge to the profitability of US pharma?!
And we don’t need to mention that in the US, the pharma lobby is considered one of the most potent lobbies, having in its pocket several lawmakers and other influential people, who circle in and out of the FDA.
The urge became even more pressing during Covid: when the ‘West’, particularly the US witnessed a crumpling of medical infra worse than third world countries. An even worse optic: a third-world country – India – was able to produce an effective vaccine within a record time, and also donated it to over 100 countries. India, known as the pharmacy of the world, was already an eyesore. But competing to be in the league of big boys was a big no-no.
And thus begins a war of narrative – from sponsored articles questioning India’s handling of COVID-19 to inflating India’s death numbers to make them look at least as bad as the West, to an orchestrated global psy-war against Indian medicines.
Again, a brown US ‘patriotic Indian’ – who is also a US citizen, becomes handy.
Introduction
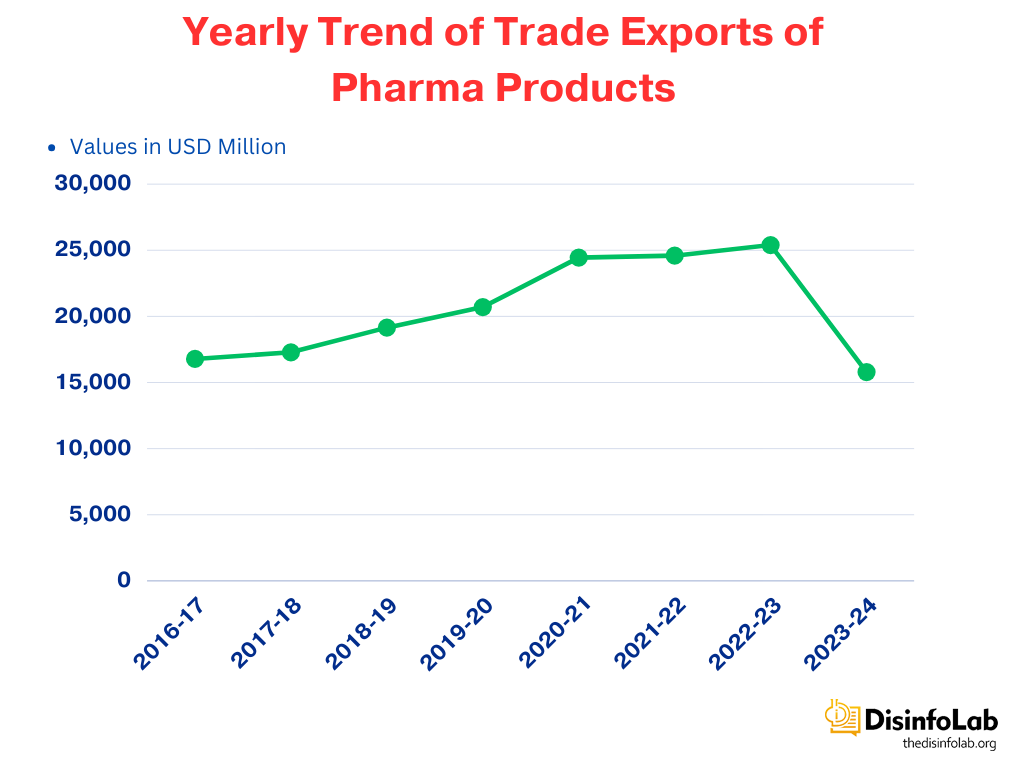
In recent decades, India has emerged as a major player in the global pharmaceutical industry, having established itself as the leading producer of low-cost, high-quality generic medicines. India manufactures about 60,000 different generic brands across 60 therapeutic categories accounting for about 20% of the share in the global pharma market [1]. In the last decade, exports of Indian Drug and Pharmaceutical products has risen by 125% [2], comprising about 5.71% of exports to around 200 countries [3]. India’s pharmaceutical exports were valued at $20 billion in 2022, and are expected to reach $30 billion by 2025.
Reasonably, India is hailed as the “Pharmacy of the World”. The country’s pharmaceutical exports are seeing rapid growth, especially in its top importers including in the North America, Europe, Africa, and Latin America. India also has a well-established manufacturing infrastructure, which is capable of producing high-quality generic drugs at a low cost. India’s pharmaceutical industry has made significant contributions to the global health landscape. For example, India was a major supplier of antiretroviral drugs to HIV/AIDS patients in Africa during the early years of the epidemic. The industry accounts for 60% of global vaccine production and supply, and it has been a major supplier of generic drugs to developing countries. In 2015, India became the first country in the world to make a generic version of the Ebola vaccine.
The COVID-19 pandemic highlighted India’s importance as a global supplier of medicines. India was one of the first countries to develop a COVID-19 vaccine, and it has since exported millions of doses to over 100 countries around the world. India has also been a major supplier of other essential medicines, such as oxygen cylinders and ventilators, during the pandemic. Many countries that were previously sceptical of Indian-made drugs have come to rely on India for essential medicines during the pandemic. India has also emerged as a key destination for clinical trials, with the potential to increase global clinical trials in the country by five times soon. This increased trust is likely to boost the growth of India’s pharmaceutical industry in the years to come.
Looking to the future, India is well-positioned to continue its growth as the “Pharmacy of the World.” The country has a number of advantages that will help it maintain its competitive edge, including a strong manufacturing base, a skilled workforce, and supportive policies. In addition, India is home to a number of innovative pharmaceutical companies that are developing new drugs and treatments. India has a population of over 1.4 billion people, and the demand for medicines is expected to grow in the coming years. The increasing focus on research and development and a growing number of partnerships with foreign companies is going to further boost the sector. A 2021 survey by the World Economic Forum found that India was ranked 11th in the world for its pharmaceutical industry. And as of 2023, it has risen to the 3rd rank.
But with increasing success, India also became a target of competing pharma eco-systems, leading to a sudden barrage of allegations on the Indian pharma industry. Such charges run the risk of damaging India’s reputation, which otherwise plays a significant part in ensuring the security of the world’s supply of drugs and enabling accessible healthcare through its cheaper pharmaceuticals.
Part I: Making of a Narrative
Chapter 1: Generic medicine export by India
India’s pharmaceutical industry is a global powerhouse, playing a vital role in providing affordable and high-quality medicines and vaccines to millions around the world. [4]:
Leading Supplier of Generic Drugs: India holds the top spot as the world’s largest supplier of generic medications, accounting for a staggering 20% of the global supply volume. This translates to making essential drugs accessible and affordable for countless individuals across the globe.
Vaccine Manufacturing Giant: India has in the last few years stepped up to 60% of the global vaccine production. It plays a crucial role in supplying life-saving vaccines like DPT, BCG, and Measles, particularly to developing countries.
Pocket-friendly and Affordable: The Indian pharmaceutical industry’s Unique Selling Proportion (USP) is price point and affordability. This has earned the moniker, “The Pharmacy of the World.”
Pharma Sub Categories (Exports)
- Ayush and Herbal products
- Bulk Drugs and drug intermediates
- Drug formulations, biological
- Surgicals
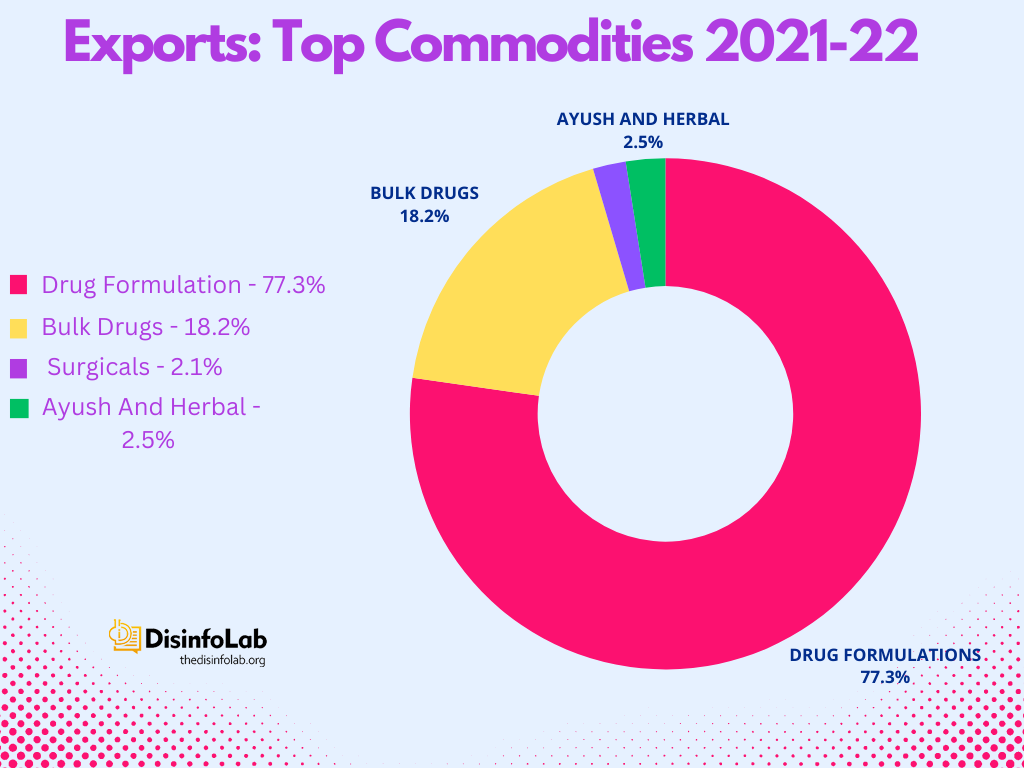
Exporting Expertise: Drug formulations and biologicals form the backbone of India’s pharmaceutical exports, contributing 75% of the total exports.
Lately, there have been a number of reports that Indian pharmaceuticals have been involved in producing and exporting substandard or falsified drugs. This has raised concerns about the quality and safety of Indian drugs and has led to some countries banning or restricting imports from India. Media reports suggest that pharmaceutical items from India may result in fatalities, multiple organ failure, and other irreversible disorders. It’s interesting to note that most of these malpractices started happening within last few years.
What’s worth noting is that, while India has been exporting medicines to almost all parts of the globe over the decades, all these reports have been emerging since last couple years, when finally, the world breathed a sigh of relief from the COVID-19 pandemic.
Chapter 2: Gambia & Maiden Pharmaceuticals
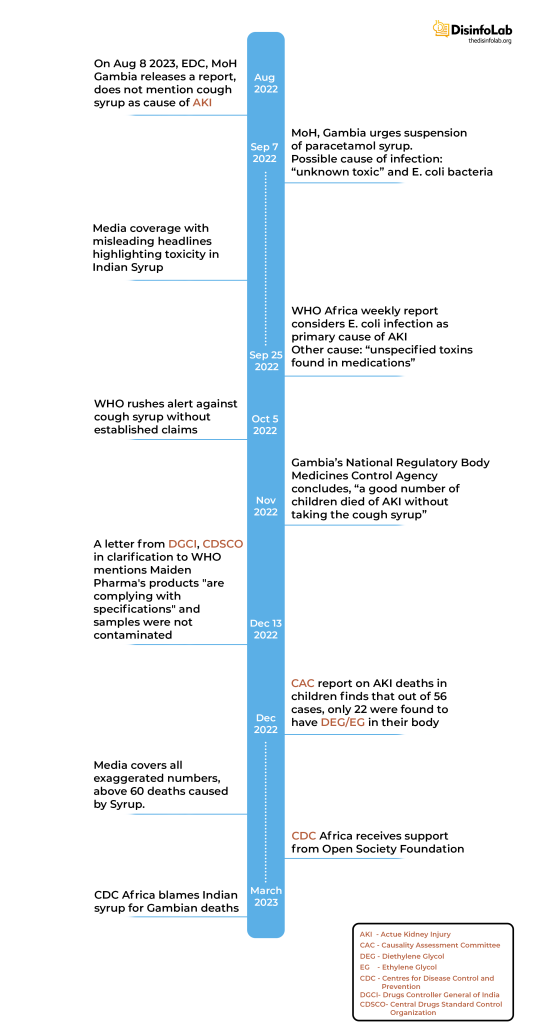
In its weekly report [6] published on September 25, 2022, WHO Africa reported, “The Gambia is experiencing an acute kidney injury (AKI) outbreak of unknown aetiology among children, identified at a Teaching Hospital since August 2022. Retrospective analysis of hospital records revealed additional cases, with the index case traced to 4 July 2022. Cases are reported from six out of seven health regions in bacteria and toxicology investigations of two samples of medicines taken by children with AKI were found to contain ethylene glycol, and diethylene glycol plus ethylene glycol, respectively. Based on the available evidence, the event has been classified as acute kidney injury secondary to E. coli infection, compounded by toxins contained in medicines.”
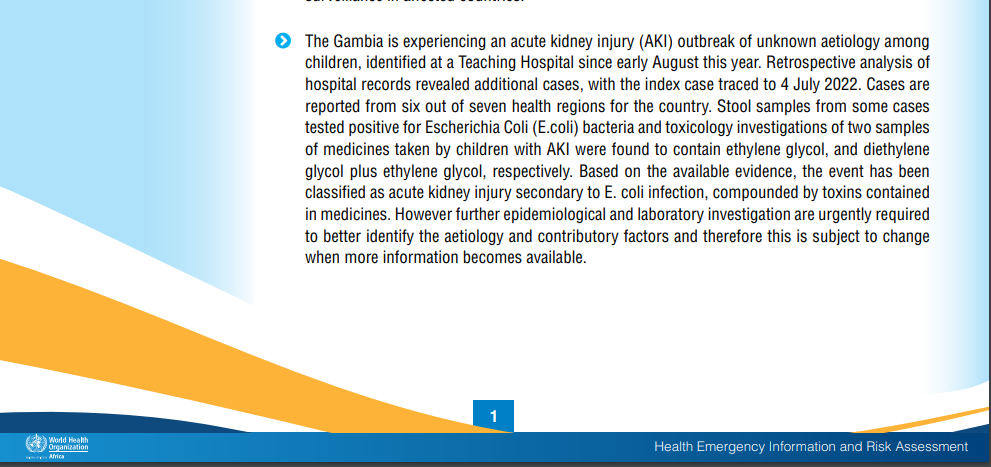
This statement suggests that after examining the existing evidence, a particular event has been identified and categorized as “acute kidney injury.” The primary cause of this kidney injury is specified as a secondary effect of an E. coli infection. In addition to the bacterial infection, the situation is further complicated by unspecified toxins found in medications.
Breaking it down:
Acute Kidney Injury (AKI): This is a sudden and rapid decline in kidney function. It can be caused by various factors, including Low blood flow to the kidneys (ischemia), Damage to the kidneys from toxins (nephrotoxicity), Inflammation of the kidneys (interstitial nephritis), obstruction of the urinary tract, health conditions like malnutrition, low blood pressure, allergic reactions, overuse of pain medications, and severe infections among others. Notably, it is most common in hospitalized patients.
Secondary to E. coli Infection: The term “secondary to” indicates that the E. coli infection is considered the underlying or primary cause of the acute kidney injury. E. coli is a type of bacteria that can cause infections, and in this case, it has led to kidney problems.
Compounded by Toxins in Medicines: The situation is made more complex or aggravated by the presence of toxins in medications. This implies that the medications, which were presumably administered to address the infection or other health issues, have contributed to the severity of the kidney injury.
A total of 75 cases with 50 deaths were reported by the Epidemic and Disease Control Unit of the Ministry of Health in Gambia. Surprisingly all these cases were reported from a single hospital in Gambia, the Edward Francis Small Teaching Hospital, Banjul. WHO reported that laboratory tests confirmed the presence of Escherichia coli (E. coli.) bacteria in 38 out of 62 samples [7] [8].
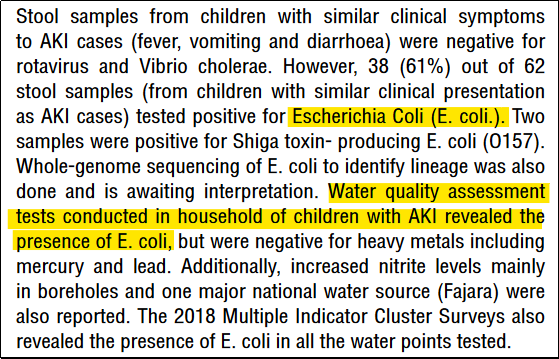
The report added that the water quality assessment of the households of the affected children also confirmed the presence of E. Coli bacteria. And since the children had been receiving treatment, the presence of paracetamol and ethylene glycol, and diethylene glycol plus ethylene glycol were also detected in two samples of medicines taken by children with AKI.
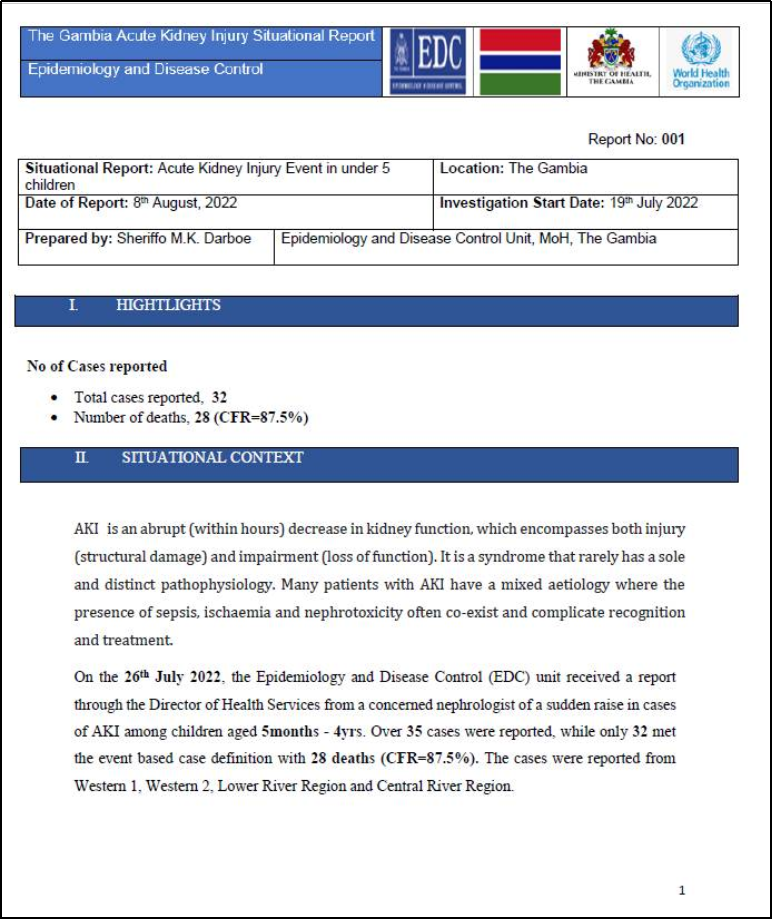
The weekly report wasn’t the first one to confirm the AKI cases. In August 2022, several reports emerged that children in The Gambia were dying due to a mysterious illness affecting their kidneys, AKI. A situational report on AKI was released by the Ministry of Health’s Epidemiology and Disease Control (EDC) Unit on August 8, 2022. As per the report, the illness was observed in 32 children under the age of 5, and it led to the deaths of 28 children [9].
A rapid increase in cases of AKI among children between the ages of 5 months and 4 years was reported to the EDC through the Director of Health Services on July 26, 2022. About the cause of AKI, the report added that the condition has “a mixed aetiology where the presence of sepsis, ischemia, and nephrotoxicity often co-exist and complicate recognition and treatment”.
This report was prepared in coordination with the attendance of WHO. There was no confirmation and linkage of the cough syrup to the AKI cases at this stage.
September 7 (2022) onwards, reports started emerging that dozens of young children (concentrated in children below the age of five) had died in The Gambia in the past three months from kidney failure. Particular paracetamol syrup has been emphasized as a potential cause, and within the context of discussing this syrup, E. coli and other potential causes were systematically downplayed. The first global portals to report this included Al Jazeera and Reuters [10] [11] [12]. These findings would later be echoed by WHO Africa report dated September 25, 2022.
In fact, as per the doctors, E. coli bacteria could also be a cause, and the most possible, as flooding had been widespread in Gambia and West Africa last year. More importantly, The Gambian health authorities said that this kind of illness often has more than one cause. While the exact cause of death was unknown, E. coli bacteria and the use of open pit latrines and open drinking wells in urban centres are also being investigated.
Despite all the above-mentioned causes, in October 2022, the World Health Organization (WHO) issued an alert about four cough syrups, namely Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup, produced by Indian drugmaker Maiden Pharmaceuticals that were allegedly linked to the deaths of children in Gambia from AKI. As per the WHO’s alert, the syrups were found to be substandard (contaminated) with diethylene glycol (DEG) and ethylene glycol (EG), toxic chemicals that are sometimes used as industrial solvents and antifreeze agents [13]. It is worth noting that the investigations were still ongoing.
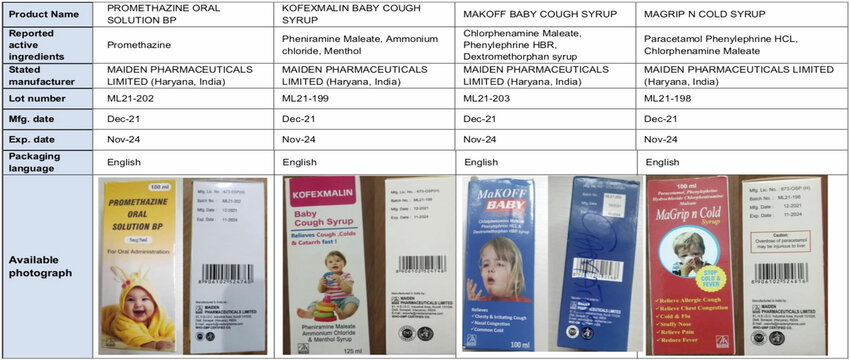
It is to be noted that until this time, no other such investigation was published which had established that it was those four cough syrups produced by Maiden Pharmaceuticals which were responsible for the AKI cases at the Banjul hospital. Even The Gambia’s Health Ministry did not release any such statement.
After WHO issued the alert, media portals started picking up the news and linked the cases to the “Indian” cough syrups. But the striking point is, that media reports did not highlight these findings of the E. coli bacteria but readily picked up the alert against the cough syrups and showcased it as the major cause of the AKI deaths in the Gambian children, implicating the entire Indian pharma industry.
After premature sirens were raised against the cough syrups attributed to the AKI deaths, India launched investigations into the Haryana-based pharmaceutical company and halted its operations [14]. Drugs Controller General of India (DCGI) V G Somani stated that WHO was asked for adequate information on the clinical examinations of the Gambia cases, which he added were not provided to India [15]. After the investigations by the Indian authorities, DCGI informed that all four cough syrups were found to be compliant with norms [16]. In a letter to the WHO dated December 13, 2022DCGI wrote that tests on samples of Maiden Pharma’s products “are complying with specifications” and showed the samples were not contaminated with ethylene glycol and diethylene glycol [17]. Indian authorities claimed that WHO had drawn premature links between the Maiden Pharmaceuticals manufactured cough syrups and the AKI deaths.
While an array of media portals claimed that the culprit behind the AKI deaths were the syrups in question, fuelled by WHO’s claim that “unacceptable levels” of diethylene glycol and ethylene glycol in the cough syrups imported from Maiden Pharmaceuticals, the Gambia authorities claimed otherwise. In early November, one month after WHO’s alert, Gambia’s national regulatory body Medicines Control Agency stated that they couldn’t conclude the medicine caused those deaths as “a good number of children” died of AKI without taking the medicines [18].
Propaganda Continues
Two reports published in December 2022 in The Gambia claimed that all the cases of AKI deaths were linked to the syrups manufactured by Maiden Pharmaceuticals. One was the Parliamentary Inquiry Report [19] on AKI published by the National Assembly in Banjul on December 20, 2022. The report itself was based on the alert issued by WHO on October 5, 2022. Even though the report admitted that nephrogenic (about tissues of the kidney) strains of E. coli were found in the stool samples of the victims, it still concluded that the cough syrups were the cause of the AKI deaths.
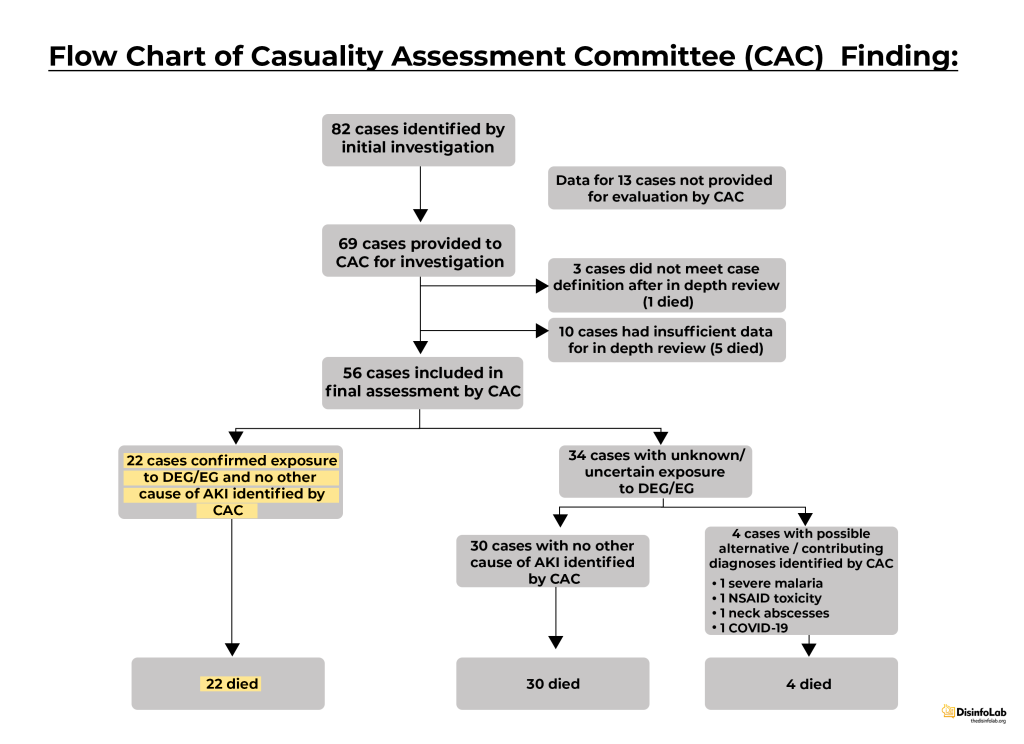
Subsequently, the Causality Assessment report [20] prepared by the Causality Assessment Committee (CAC) formed to investigate deaths caused by the AKI outbreak reported 82 cases of AKI in children between June 2022- October 2022. After excluding cases with insufficient data or that did not meet the case definition, 56 cases were assessed in depth as claimed in the report. Important facts to note: Based on the detailed review of 56 cases, 22 were assessed as very likely to have died due to diethylene glycol (DEG) and ethylene glycol (EG) poisoning from the analysed oral liquid formulations. The remaining 34 cases were found no cause for AKI but suspected to be contaminated but with uncertain and unknown exposure.
Incidentally, autopsies report of the two children revealed that they were already suffering from other health issues and the presence of DEG/EG couldn’t be confirmed.
Third Country Testing
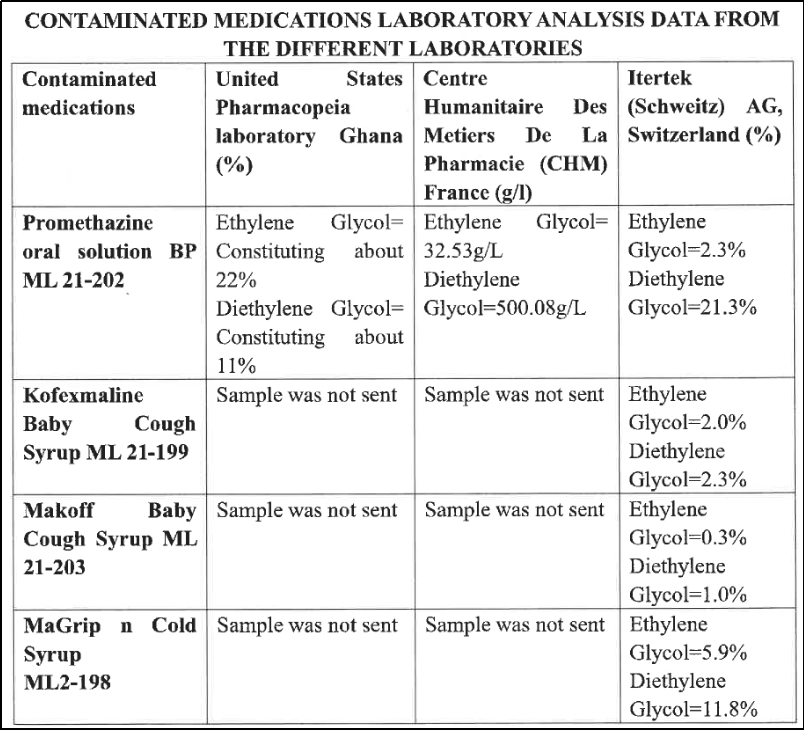
The substances DEG and EG were alleged to have been detected in four medicinal products ingested by the children, all manufactured by one pharmaceutical company (Maiden Pharmaceuticals). As per the report, these syrups were sent to three countries for testing: France, Ghana, and Switzerland, all of whom, it claimed, confirmed the presence of DEG/EG in all four samples.
However, upon examination it was found that only one syrup was confirmed to be contaminated by DEG/EG in all three places while the samples of the other three were not sent to other countries except Switzerland!
Based on these observations, and only 22 cases found to be ‘somehow’ linked to contamination by DEG/EG. But the report concluded that the outbreak of AKI in children in The Gambia was caused by DEG/EG poisoning from four medicinal products manufactured by one pharmaceutical company!
Twist in the Tale: Enters Soros
It was by now clear that the claims of contaminated medicines were not substantial, and that WHO had misled with the premature claims. However, yet another report made headlines holding the Maiden Pharmaceuticals cough syrups responsible for the Gambia deaths – a report by Africa Centres for Disease Control and Prevention or CDC Africa [21].
CDC Africa on March 3, 2023, published an ‘investigative report’, which suggested that Maiden Pharmaceuticals’ cough syrups contained unacceptable levels of ethylene glycol and diethylene glycol. Here again, without mentioning their quantities. The report concluded that Maiden Pharmaceuticals’ cough syrups were not properly manufactured. It further stated that the syrups were not tested for ethylene glycol or diethylene glycol before they were released for sale.
Coincidentally, in 2020, CDC Africa received USD500,000 from George Soros’ Open Society Foundations (OSF) for a period of two years! [22]
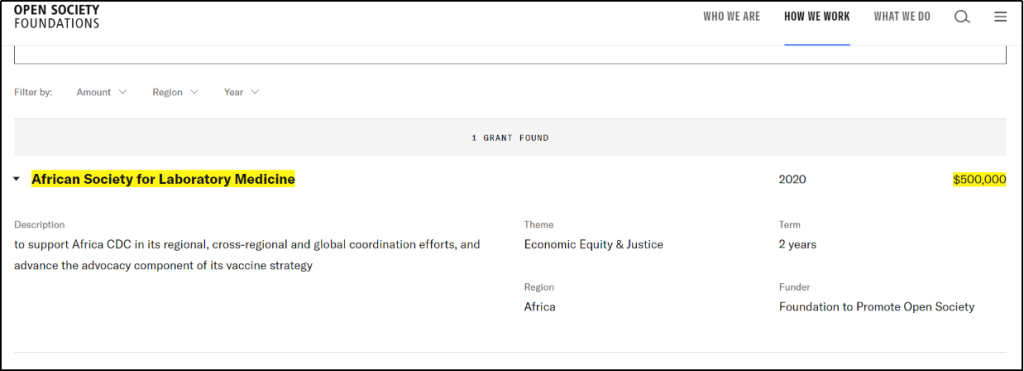
Notably, after all this reporting, WHO itself stated in June 2023, that the United Nations body is yet to confirm a link between the syrup and the AKI deaths [23]. To this date, no report or investigation has proven the syrups were the definitive cause of the tragic death of the Gambian children who died of AKI. But thanks to the WHO’s premature report and allegations by Gambian authorities coupled with scores of media reports, the case has been plastered on Indian pharmacy.
The Narrative:
Even if one were to presume that the medicines were contaminated, it was ONE cough-syrup from millions of similar exports worldwide. However, the story was not about one medicine- it was used to paint entire Indian pharmacy in black, in an effort to dent India’s image of the “Pharmacy of the World”.
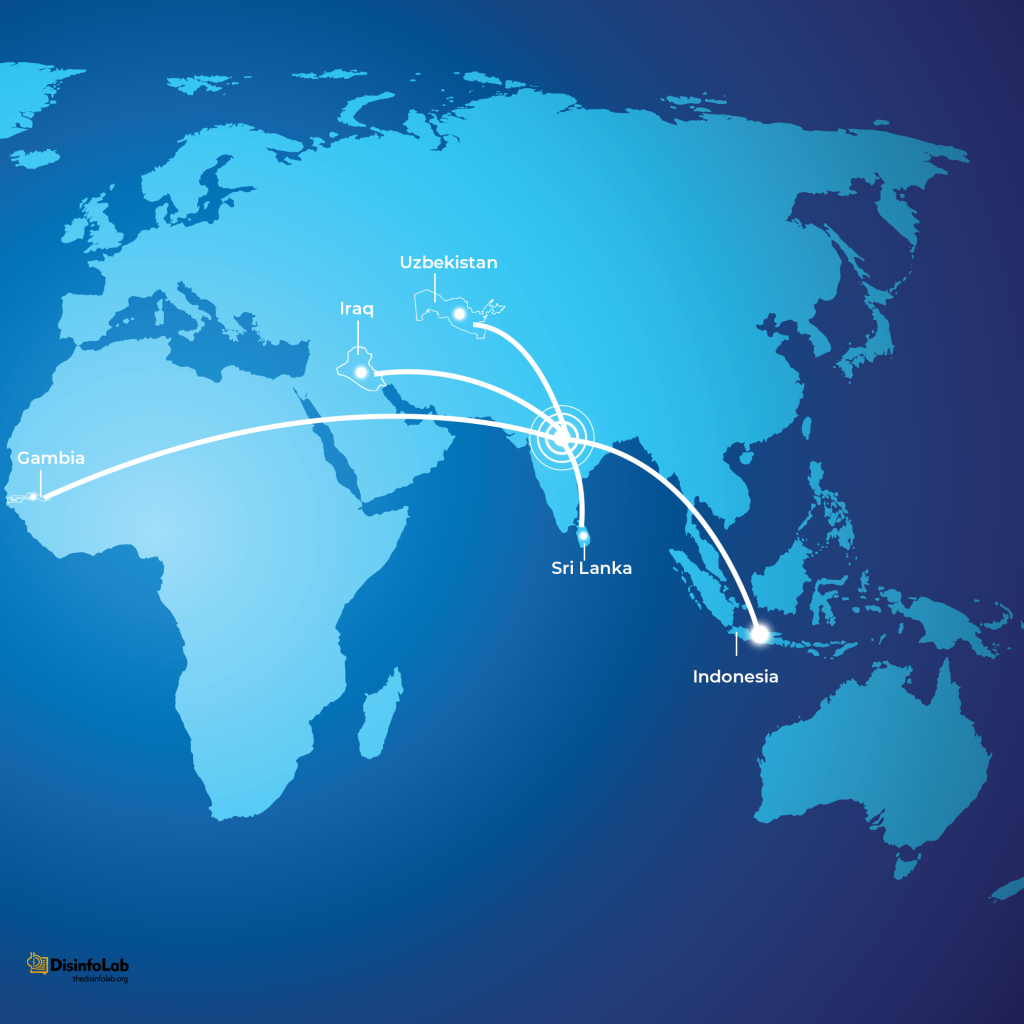
Chapter 3: The Sri Lanka, Indonesia, Iraq and so on
Another incident that targeted the image of Indian pharma is the case of eye drops, which was linked to vision loss and other complications in hospitalized patients in Sri Lanka. The company implicated in this case was Gujarat-based Indiana Ophthalmics. The company was accused by the Sri Lankan government of supplying eyedrops that caused eye infections in over 30 people [24][25].
On May 12, 2023, cases of vision loss were reported at three government hospitals in Sri Lanka, namely National Eye Hospital in Colombo, Government Teaching Hospital, Batticaloa, and General Hospital in Nuwara Eliya City, due to the purported use of ophthalmic methylprednisolone eye drops imported from an Indian company Gujarat-based Indiana Ophthalmics. Purportedly, the eye drops were found to be infected by Burkholderia capacia, a bacterium that allegedly caused vision loss in at least eight patients. As per the doctors, the cases were first reported in early April [26].
Note: Methylprednisolone is a prescribed medication in ophthalmology for the treatment of severe allergic and inflammatory conditions of the eye and its adnexa, including allergic conjunctivitis, uveitis, scleritis, chorioretinitis, iritis, and iridocyclitis. Methylprednisolone and its derivatives, methylprednisolone acetate succinate and methylprednisolone sodium, are synthetic glucocorticoids with an intermediate action that are mostly employed to inhibit the immune system. However, the allegations made by the media were classified under the drug’s adverse effects, which had nothing to do with manufacturing. The main negative effects of glucocorticoids are maybe brought about by their hormonal actions, which cause cataract growth, elevated intraocular pressure, and exophthalmos to appear [27].

Sri Lanka first suspended the use of eye drops in April after three patients who had undergone eye surgery suffered from complications. Alleging that the Indiana-Ophthalmics eyedrops were responsible for the cases, Sri Lanka withdrew the eye drops and blacklisted them [28].
As soon as allegations started against the Indian pharmaceutical industry, scores of media reports started making the same allegations, linking the case not simply to the eye drops or the company allegedly responsible, but this time again the whole narrative was set to show Indian pharma being responsible. Notably, at this stage, no in-depth investigations or analyses were carried out that could ascertain the eye drops to be the cause behind the complications.
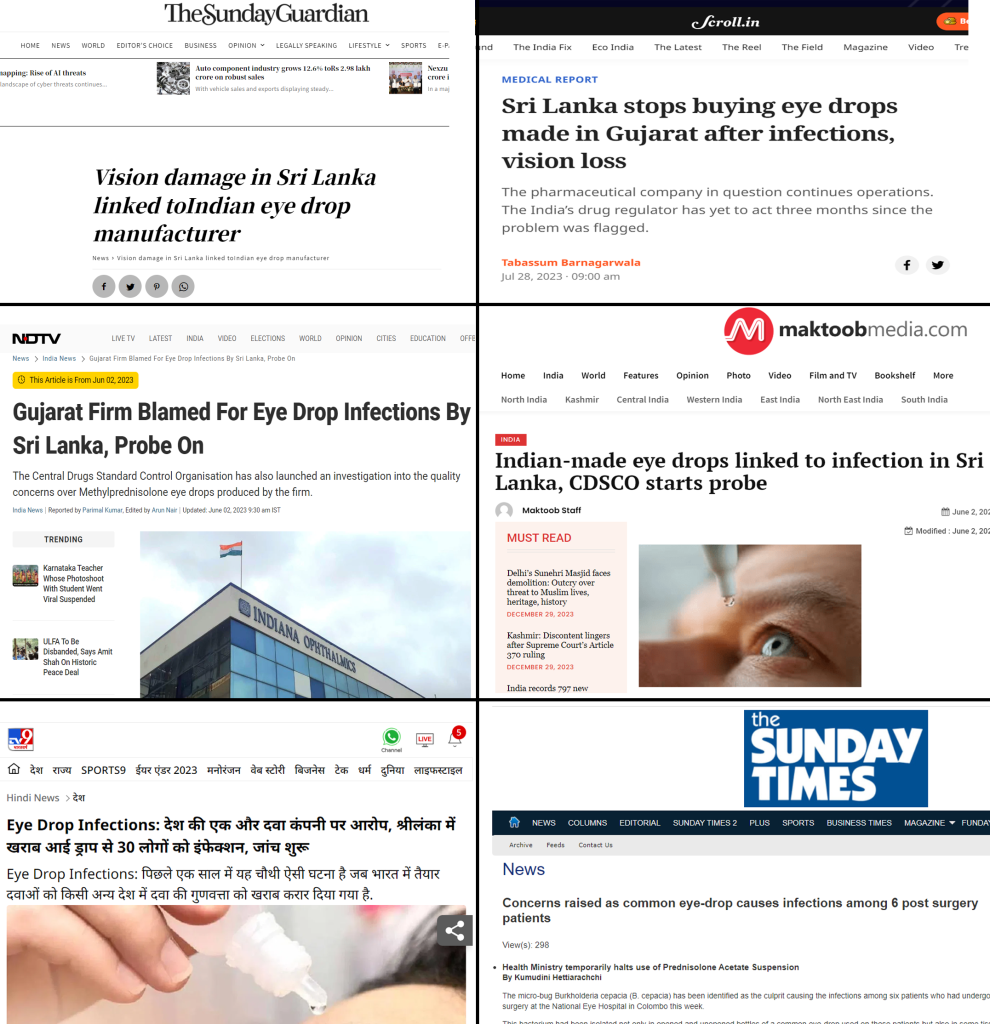
After allegations by the Sri Lankan authorities, India’s drug regulatory authority, the Central Drugs Standard Control Organization (CDSCO) initiated a probe into the matter in early June [29]. According to media reports, the bacterial infection found in the eyes of Sri Lankan patients is of the “gram-negative type,” an MDR group of bacteria that is typically present in hospitals and operating rooms, according to H.G. Koshia, the commissioner of the Gujarat Food and Drugs Control Administration [30]. Despite this, by July 28, the Sri Lankan Government linked as many as 50 cases of eye complications to the eye drops and stopped buying the drops [31]. This time around, however, even WHO noted that there were no side effects connected to the ointment [32].
Moreover, Indiana Opthalmics claims on its homepage to be among the country’s largest eyedrops and eye ointment makers, with a WHO-GMP (Good Manufacturing Practices) certification since 2007 [33].

But the damage was done and while a slew of media reported on the allegations, that wasn’t the case after the clarification on the eye drops. The media reports did not even bother to check these basic facts about the contamination.
Indonesia’s Cough Syrup Ban and Role of Media
In 2022, a devastating public health crisis unfolded in Indonesia when over 200 children perished due to AKI, allegedly linked to contaminated cough syrups. During the investigation, Indonesian authorities found that a prime accused, drugmaker Afi Farma, used industrial-grade solvent material, Ethylene Glycol and Diethylene Glycol, with toxin concentrations of up to 99% in 70 batches of the syrups [34].
While the tragedy stemmed from tainted ingredients within Indonesia’s own pharmaceutical industry, a concerning narrative emerged in some media outlets – the unjustified implication of Indian pharmaceutical companies. Incidentally, around the same time the Gambia cough syrup tragedy had taken place allegedly linked to Indian pharmaceutical company Maiden Pharmaceuticals. Several media portals used sensational headlines like the headline used by Times Now “Indonesia bans ingredients of Indian cough syrup linked to Gambia child deaths. [35]”
Despite evidence clearing Indian involvement, news reports often used suggestive headlines, misleading visuals, and a lack of clarity, while linking both the Indonesia and Gambia cases, creating a false impression of Indian culpability, and fuelling negative perceptions of Indian pharmaceuticals. Global media NYTimes [36], CNBC 18 [37], Reuters played an important role in adding weightage to the narrative already being churned in Indonesia’s case.

These almost identical headlines could not be explained away simply for click-bait. It was a most coordinated narrative, if there was one and needs to be investigated deeply.
Despite the WHO’s call for investigation and evidences coming up after the investigation leading to no linkages between the cough syrups in Indonesia with India. Many media portals which had linked two cases previously did not do a follow-up story after WHO’s clarification [38].
Cases Reported in Countries Against Indian Syrups
Part II: Unmasking the Narrative Builders
Chapter 4: the ‘Indian American’ Truth Pill
In October 2022, when the buzz on Gambia was at its peak in both Indian and Western media, a book was launched The Truth Pill: The Myth of Drug Regulation in India on October 10, 2022, which dives into the alleged shortcomings of India’s drug regulatory framework – written by Dinesh Singh Thakur and Prashant Reddy. The book critiques the weaknesses within India’s drug regulation system, claiming it fails to adequately safeguard public health in hindsight. Thakur and Reddy argue that despite the perception of India as a global leader in pharmaceuticals, significant problems exist regarding manufacturing practices, clinical trials, and government oversight. Incidentally, Thakur, the US based pharma expert, became the go to quote for most of the Indian media reporting. The manufactured narrative in Gambia case provided a perfect backdrop for the bool launch. Coincidence.
The Authors


Prashant Thikkavarapu Reddy is a lawyer and an author. He earned his BA LLB (Hons.) from the National Law School of India University in 2008 and an LLM from Stanford University. He has worked as a Research Associate at the recently closed Applied Research Centre for Intellectual Assets and the Law in Asia, School of Law, Singapore Management University (2016-17). In 2018, he joined the National Academy for Legal Studies and Research, Hyderabad as an assistant professor where he taught Intellectual Property Rights and Administrative Law. He now practices independently [39]. In 2018 he also joined Dinesh Thakur as the Director of one of his organizations which will be elaborated in subsequent sections.
Ever since its launch, the book as well as its authors have been quoted by numerous Indian as well as international media portals. Notably, all the media articles that reported about the new book back then were all headlined around the Gambia incident projecting Thakur and Reddy as ‘health activists’. Global media portals including ‘The Independent UK, BBC, Forbes, Al Jazeera, and Guardian also began promoting Thakur and his book while portraying him as an expert and a public health activist. Thakur also made the most of this manufactured tragedy i.e., the Gambia incident, to promote his new book on his social media handles [40].
Incidentally, while most of Indian news media started quoting Thakur after Gambia episode and after his book was launched, The Wire in India had an article ready even before the book launch.
Dinesh Singh Thakur
Dinesh Singh Thakur is best known for his whistleblowing saga against the Indian pharmaceutical giant Ranbaxy Laboratories, which was implicated by the Food and Drug Administration (FDA) of the US for falsifying drug data and violating ‘Good Manufacturing Practices’ (GMP) in 2013. Ranbaxy pled guilty to the charges and paid the US government a fine of USD 500 million to settle the criminal and civil lawsuit [41]. Thakur pocketed USD 48 million from the Ranbaxy lawsuit for his whistle-blower complaint.
Background
As per his website, Dinesh Singh Thakur is a public health activist, trained chemical engineer, an expert and accomplished entrepreneur in pharmaceuticals, biomedical product development, drug regulation, and information technology [42].
Thakur is a Chemical engineering graduate from Osmania University, Hyderabad (India). During his college days in India, he applied for Graduate Record Examination (GRE) programs in the US and eventually earned a scholarship from the University of Hampshire. From there, he completed his Master of Science in Chemical Engineering (1990-92) [43].
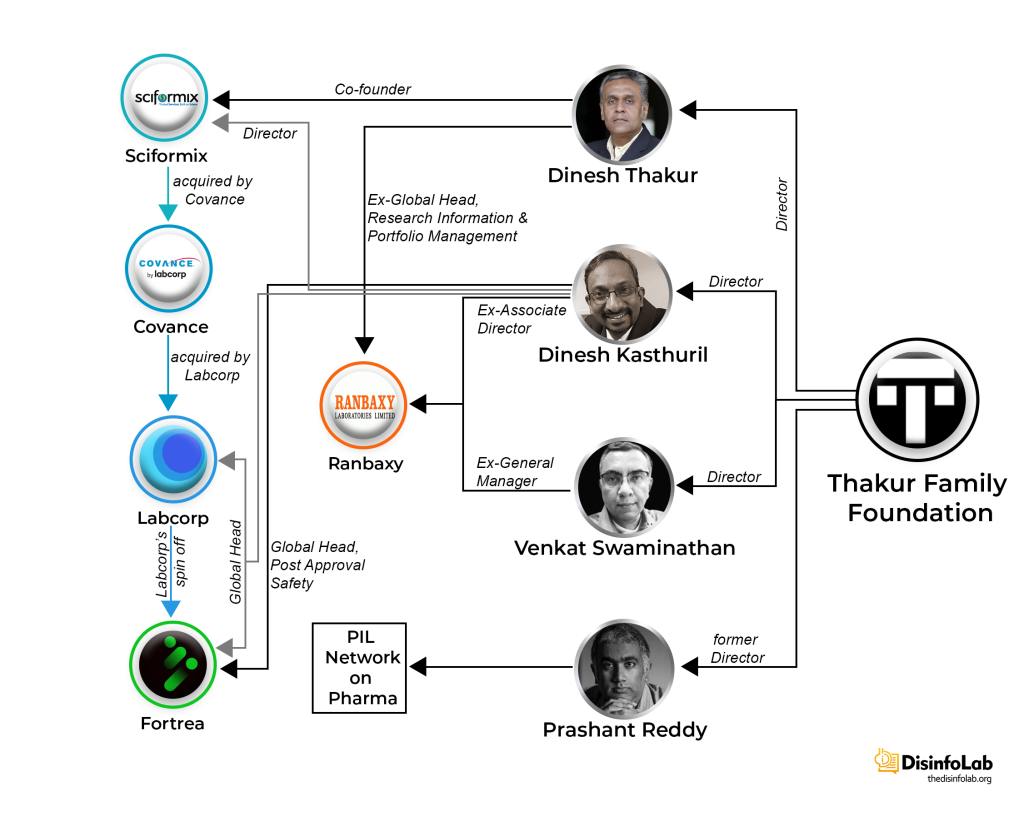
Currently, Thakur lives in St. Petersburg, Florida, and is the founder and Director of an independent funding organization Thakur Family Foundation (TFF) which he founded in 2018.
[Note: There are no social media traces of Thakur’s life before 2013- likely cleaned. He created his account on X (Twitter) in February 2013, followed by registering a website domain under his name which is accessible at www.dineshthakur.com. [44]]
Before his whistle-blowing incident, Thakur had been working in several companies in the US including his first job as a research associate at the Genetics Institute (now Pfizer) for a year between 1992-93. He then moved to a global biopharmaceutical company Bristol-Myers Squibb (BMS), headquartered in Princeton, New Jersey where he worked for 10 years (1993-2003).
While being employed at the BMS, Thakur also pursued his double Master’s program in Computer Engineering in 1996 from Syracuse University, the fees of which were borne by his employer.
However, after serving for ten years at BMS, Thakur returned to India in 2003 after one of his seniors at BMS, Rashmi Barbhaiya convinced him to join then-India’s largest generic drug manufacturing giant Ranbaxy Laboratories (in Gurgaon) as Director and global head of research information & portfolio management.
Note: The first company that Thakur worked in, Genetics Institute was engaged in the research and development of drugs and therapies based on genetic engineering. The company was fully acquired by another company, Wyeth Nutritionals in 1996 [45]. Thirteen years later, in 2009, Thakur’s first company transitioned into a larger pharmaceutical brand in the US i.e., by Pfizer which in turn was acquired and Wyeth was acquired by Pfizer in 2009 for USD 68 billion [46].
He started gaining prominence in 2019 and most recently during the Gambia case when he released his book “The Truth Pill”. Thakur runs a grant-making organization in the US and commissions many journalists, NGOs, and, Universities based in India under the Public health, Civil liberties, and Right to Information (RTI) category which will be discussed subsequently in this report.
Chapter 5: Dinesh Thakur & Ranbaxy Episode
As per the book “Bottle of Lies: The Inside Story of the Generic Drug Boom”, Thakur visited India in 2002 during summer vacation and stopped by Ranbaxy’s research center in Gurgaon as he was seeking a job switch from BMS. The next year, he returned to India and in 2003, leaving a cushy job at Bristol Myers Squibb (BMS).
In 2003, Thakur, along with two of his colleagues at BMS, Dinesh Kasthuril and Venkat Swaminathan came to India and joined Ranbaxy. Dinesh Kasthuril joined Ranbaxy as Associate Director, while Venkat Swaminathan joined as the General Manager [47][48]. Both of his colleagues at BMS and then Ranbaxy further went on to become part of Thakur’s grant-making Thakur Family Foundation (TFF), which will be elaborated later in the report.

In 2004, Thakur was delegated the job of investigating the data on the generic ARV drugs (or antiretroviral drugs to treat HIV/AIDS) drafted by the WHO, following their inspection conducted at the Vimta Labs, hired by Ranbaxy to administer clinical tests of their AIDS medicine.
The book claimed that many of the patients who had been enrolled by Vimta for the ARV drug test did not exist. Both Thakur and his immediate boss Rajinder Kumar unearthed the fraud. They wanted the medicine to be taken off the market, though the company apparently resisted.
Therefore, since his employer didn’t choose to do anything about the data falsification charges accounted for in the WHO report about the ARV drug. In 2005, Thakur left Ranbaxy and decided to expose the company’s alleged fraud. After resigning from Ranbaxy, Thakur contacted the United States Food and Drug Administration (FDA) (in the same year) for whistle-blowing on Ranbaxy’s data falsifying practices. Thakur worked closely with the FDA to expose the Ranbaxy adulteration.
There are many emotional twists to his ‘return to India to serve’ story.
As accounted in the book, at that time Thakur’s son was suffering from a serious eye infection. The paediatrician prescribed him an antibiotic, Amoxyclav by Ranbaxy which didn’t seem to work on his son. Thakur turned to a different medicine; antibiotic manufactured by the US-based big pharma company GlaxoSmithKline which turned out to be effective. That is when Thakur determined that his family wouldn’t take Ranbaxy medicines anymore until he found out the truth.
It was rather touching. He was so fond of India that he happily renounced Indian citizenship, and acquired American. He comes to work for a company that was going to pose major threats to the US mega firms. And all he worked for few years, only to file a complaint against the Indian company to US FDA. Incidentally, he came to India with some pals, who also worked for the same company, but in different departments, and returned to the US like him, after ‘serving’ mother India. Incidentally, they joined Thakur’s firm as well. And while US pharma holds sway over the US FDA, Thakur and company wants us to believe that the whole story was merely lack of following good practices.
India must be proud of producing such patriots.

Pfizer vs Ranbaxy Lipitor Drug Litigation
In the early 2000s, a David vs Goliath type battle emerged between world’s leading big pharma Pfizer and India 3rd largest generic drug manufacturer Ranbaxy Laboratories. The legal battle back then was over world’s largest selling cholesterol medication. The litigation battle began once Ranbaxy chose to manufacture the generic and affordable version of the Lipitor drug which Pfizer had the monopoly of, and had been making billions from its sale worldwide including in the US.
Inputs for Timeline taken from: –
(UNITED STATES COURT OF APPEALS Nos. 14-4202, 14-4203, 14-4204, 14-4205, 14-4206, & 14-4602 IN RE: LIPITOR ANTITRUST LITIGATION FOR THE THIRD CIRCUIT)
&
US District Court For The District Of New Jersey Case: Pfizer Inc., Pfizer Pharmaceuticals LLC, Pfizer Ltd, CP Pharmaceuticals, Pfizer Ireland, Warner-Lambert Company, Warner Lambert Company LLC, and Warner-Lambert Export, Ltd vs Ranbaxy Laboratories Limited, and Ranbaxy Inc.,
Lipitor Litigation Timeline
1987: the U.S. Patent and Trademark Office (PTO) granted Pfizer the original patent for Lipitor. That patent—designated U.S. Patent No. 4,681,893 (the ‘893 Patent)—claimed protection for atorvastatin, Lipitor’s active ingredient. Although initially set to expire on May 30, 2006, the ‘893 patent received an extension from the FDA, lengthening the patent’s term through March 24, 2010.
1993 (December): Pfizer obtained additional, follow-on patent protection for Lipitor when the PTO issued U.S. Patent No. 5,273,995 (the ‘995 Patent). That patent claimed protection for atorvastatin calcium, the specific salt form of the active atorvastatin molecule in Lipitor.
1997: After obtaining the ‘893 and ‘995 Patents, Pfizer launched Lipitor in 1997. Pfizer through Parke-Davis Pharmaceutical (under Warner-Lambert company), which had formulated atorvastatin calcium, (an active ingredient in the cholesterol medication) got together to sell the drug under the trade brand name Lipitor. The application of the Lipitor drug was to reduce the level of LDL cholesterol in the bloodstream. By 1999, Lipitor became the world’s best-selling drug, generating billions of dollars of revenue for the company.
Ranbaxy’s Attempt I:
2002: Ranbaxy Laboratories reported to have developed the generic version of Lipitor. Therefore, in August, Ranbaxy secured the first-filer position for a generic Lipitor Abbreviated New Drug Application (ANDA) in August 2002, gaining a strategic advantage in the race to market a lower-cost alternative. It made Ranbaxy the first company to file for an application with the FDA for production of generic version of atorvastatin.
This posed a challenge to Pfizer, which was possessing patent rights for Lipitor.
[Note: ANDA is a submission made to the FDA in the United States to seek approval to market a generic version of a brand-name drug that is already approved [49].]
2002-03: Shortly after, Ranbaxy notified Pfizer regarding the ANDA filing by the latter to the Food and Drug Administration (FDA) for the approval of the generic version of Lipitor drug for commercial manufacture and sale in the US market and worldwide prior to the expiration of ‘893 patent held by Pfizer.
2003: On February 23, Pfizer filed a patent infringement lawsuit against Ranbaxy in the United States District Court of Delaware. The company in its lawsuit claimed that the Indian generic drug manufacturer was infringing several claims of the ‘893 patent. The case came to be known as “Lipitor® Litigation” [50]. As a result of Pfizer’s lawsuit, the FDA withheld approval of Ranbaxy’s ANDA for 30 months pursuant to the Hatch-Waxman Act.
In return, Ranbaxy filed several counterclaims while responding to the claims made by Pfizer in their lawsuit.
[Note: 45 days are given to the NDA holder or patent owner (Pfizer here) to challenge the ANDA applicant & even initiate a patent infringement action.]
Counter Accusation by Ranbaxy: Ranbaxy claimed that Pfizer procured the ‘995 Patent via resorting to fraudulent practices further alleged that the company submitted false and misleading data to the PTO to in order to obtain the patent ownership for the cholesterol medication.
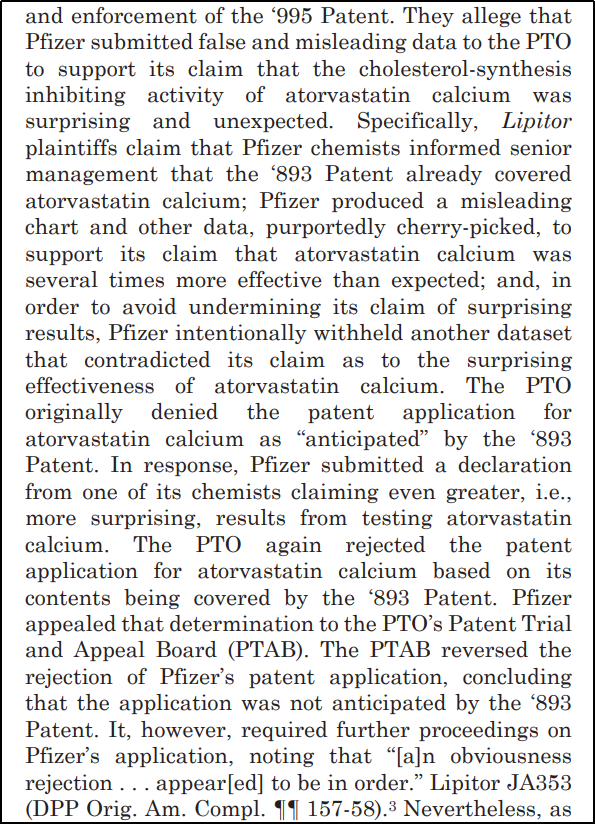
2005: The litigation battle escalated. This dispute extended to various patents. The patent litigation fight was not limited to the US, but also started in Europe and Australia. But the odds were not in favour. An Indian pharma company taking a US giant to court in the US itself, where Pharma lobby is second only to the defence lobby. In December, a US District Court ruled against Ranbaxy. The Indian firm tried appeal to the United States Court of Appeals for the Federal Court, but remained unsuccessful.
In July 2005, as the 30-month statutory window barring Ranbaxy to sell its generic version headed towards culmination, Pfizer filed a citizen petition with the FDA citing that the amorphous non-crystalline form of atorvastatin used in generic Lipitor may be of “inferior in quality” in comparison to the NDA’s Lipitor’s crystalline form. In response, Ranbaxy claimed the citizen petition as a sham and alleged that Pfizer’s citizen petition lacked evidences to support its claims.
In May 2006, FDA responded to Pfizer’s citizen petition and stated that the drug regulatory body hadn’t reached a decision and further review was needed given the complexity of the issue raised by the company in the petition. (Later on, in 2011, the FDA rejected the citizen petition.)
On November 7, 2006, the final judgement took place at the district court on the Lipitor litigation which announced the verdict in favour of Pfizer and that Ranbaxy would not launch its generic version until the expiration of Pfizer’s extended patent expiration date which was September 24, 2009, followed by a further entitlement of six months extension to March 2010 as the company planned to test the drug’ effects on children [51].
Lipitor was a blockbuster drug by Pfizer. It generated a sales turnover of close to US$13 billion in 2006, and was the largest selling drug for the company and in the global pharmaceutical market. More than 60% of its sale was from the US alone [52]. It would be hard to let go of the profits, even if it would have benefitted millions of Americans with affordable drugs.
Ranbaxy’s Attempt II:
2007: On January 24, Ranbaxy wrote a letter to Pfizer to notify them about their ANDA filing and seeking approval from the FDA to manufacture and sell their new product containing amlodipine besylate and atorvastatin calcium before the expiration of Pfizer’s extended ‘893 patent.
In March 2008, Pfizer again sued Ranbaxy in District of Delaware citing- Ranbaxy’s generic drug would infringe Pfizer’s two Lipitor-related process patents.
Counter Accusation by Ranbaxy: Ranbaxy called it a sham. Ranbaxy further added that US pharma giant knew that their generic product wouldn’t infringe Pfizer’s patents and that the pharma giant was using that lawsuit to enter into a reverse payment settlement agreement.
On June 18, 2008, both Pfizer and Ranbaxy reached a global settlement agreement, ending the legal disputes. It was after the U.S. District Court for the District of Delaware
passed judgement in favour of Pfizer over Lipitor patent ownership. The subsequent settlement in 2008 required Pfizer to drop damages claim against Ranbaxy. The District Court ordered Ranbaxy to refrain from launching the generic version of Lipitor until Pfizer’s patent expires i.e., until November 30, 2011 [53].
On November 30, 2011 Pfizer’s Lipitor patent expired. The company made revenue worth US$130 billion since the drug was launched in 1997 [54].
On December 1, 2011, Ranbaxy launched its first generic version of the Cholesterol-lowering Lipitor. Ranbaxy got the market exclusivity for 180 days to sell its generic medicine [55].
Note: Under the US law- Drug Price Competition and Patent Term Restoration Act of 1984 (the Hatch-Waxman Amendments), the first generic to enter the market gets the market exclusivity for 180 days [56]. Ranbaxy Laboratories managed to capture close to 2.5 % of the generic Lipitor’s market share in the U.S within the first few days of the launch of its generic version of cholesterol medication.
Thakur Coincidence II:
It is exactly around the time when Ranbaxy made its first attempt at Lipitor in 2002 that the ‘love of India’ erupted in Thakur’s heart (who had become US citizen) and next year came to join Ranbaxy. Within two years, he went back, and set to destroy the company.
It was also the time when Thakur’s whistle-blowing case against Ranbaxy with the help of the USFDA was in its final days. In 2013, after fighting for almost nine years, Ranbaxy group pled guilty. The company was fined USD 500 million and was forced to take several corrective actions [57]. Thakur received USD 48.6 million for his whistleblowing.
After the incident, one of Ranbaxy’s largest investors Daiichi Sankyo which had acquired 63/9 % for USD 4.2 billion in 2008 sold its shares to India’s Sun Pharmaceuticals in 2015 [58]. The buyout of Ranbaxy to Sun Pharma was valued at USD 3.2 billion plus the USD 800 million debt absorbed by the latter which totalled up to USD 4 billion.
All about Narrative
There are conflicting views about the actual scale of malpractice by Ranbaxy. Some experts have claimed that most of it emanated from the gap in practices in the US and India. Also, the accusations were more of a nature of wrong documentations, which was portrayed as bad quality medicine [59].
However, lets grant that Ranbaxy medicine was indeed substandard, and the company paid a price for it. But the narrative was NOT about a particular company making a mistake. Narrative was about bad ‘Indian pharma’. Thakur used this story to paint the entire pharma sector in India in dark shades. His particular target has been generic medicines.
What he, and other story-tellers, forget to mention that Ranbaxy saga was par for the course for pharma industry. An unfortunate situation yes, but not exclusive to India. The same year, when Ranbaxy was made to pay USD 500 million as penalty, other US companies including Pfizer, Moderna etc, were made to pay similar penalties.
But it never became story of bad US pharma. Only, bad India pharma. Orchestrated by Indian origin American whistle-blower. And the story telling continues – from Gambia to India. We will return to it shortly.
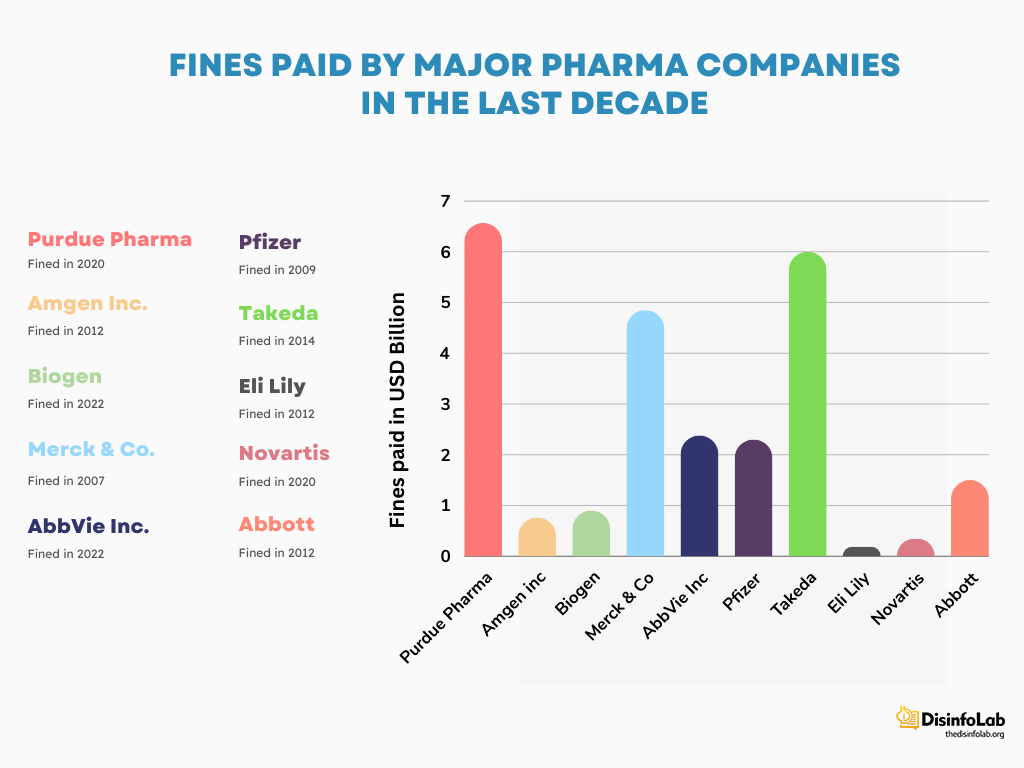
Fines Paid by Other Pharmaceutical Companies
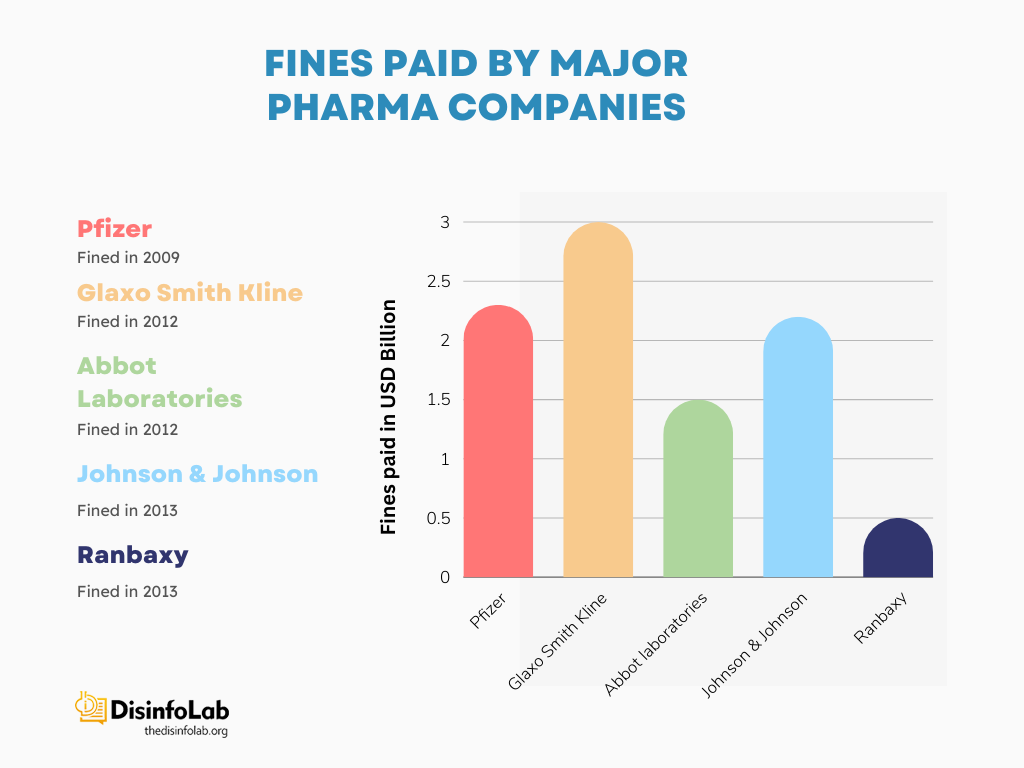
- In 2009, Pfizer and its subsidiary Pharmacia & Upjohn agreed to pay USD 2.3 billion to the U.S. Department of Justice illegal promotion of several of Pfizer’s drugs. Other allegations included off-label marketing kickbacks to physicians and pharmacies, and misrepresenting safety data to the FDA. Pfizer’s fine settlement at that time became the largest fraud settlement in the pharma sector [60][61].
- In 2012, Glaxo-Smith-Kline pleaded guilty and paid a fine of USD 3 billion to resolve the allegations it had been found practicing. Glaxo-Smith-Kline faced three-pronged criminal charge: two counts for introducing unauthorized versions of the drugs Paxil and Wellbutrin into interstate commerce, and one count for failing to disclose safety data about Avandia to the FDA. GlaxoSmithKline pleaded guilty to misbranding Paxil and Wellbutrin and hiding Avandia safety data [62].
- In 2012, Abbott Laboratories pled guilty to unlawful promotion of the prescription drug Depakote for uses that were not approved as safe and effective by the US FDA. Abbott Labs was made to pay USD 1.5 million as a fine to settle the accusations of fraudulent practices. At that time, it became the second-largest settlement ever paid by a drug company in the United States [63].
- In 2013, Johnson & Johnson agreed to pay USD 2.2 billion to settle allegations of illegal marketing and kickbacks related to several of its prescription drugs. J&J was accused of promoting antipsychotic drugs Risperdal and Invega for uses not approved by the Food and Drug Administration (FDA), also termed as “off-label marketing”.
- J&J paid kickbacks to physicians and pharmacies to prescribe their Risperdal and Invega. Lastly, J&J was also accused of failing to report important safety data about the heart failure drug Natrecor to the FDA. The fine settlement of J&J became one of the largest healthcare fraud settlements in U.S. pharmaceutical history [64].
The US FDA – Pharma Circle & Thakur
In fact, US FDA has a revolving door with major US pharma companies. The companies they are supposed to monitor. And in case of a conflict with a foreign firm, may also be the arbiter.
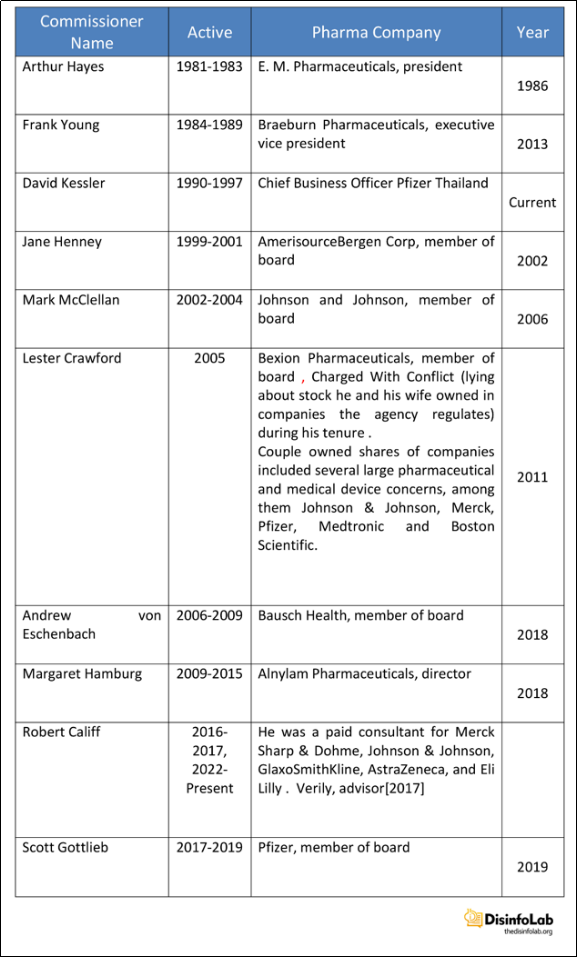
What is Next? In 2017, the American Enterprise Institute, a Washington DC-based public policy think tank comprising of academicians, scholars, and professionals published a report [65] titled India’s Dodgy Pharmacy [66] by Roger Bate, who recommended Dinesh Thakur for his appointment at the top FDA job in India to then FDA commissioner, Scott Gottlieb. Incidentally, both Gottlieb and Bate have worked together at AEI. This remains a development to watch.

Chapter 6: Friends ‘In-Deed’
Thakur was not the only one to have resigned from Ranbaxy in 2005. All his colleagues who were at Bristol Myers Squibb (BMS) resigned from Ranbaxy around the same time.
Sciformix Corporation
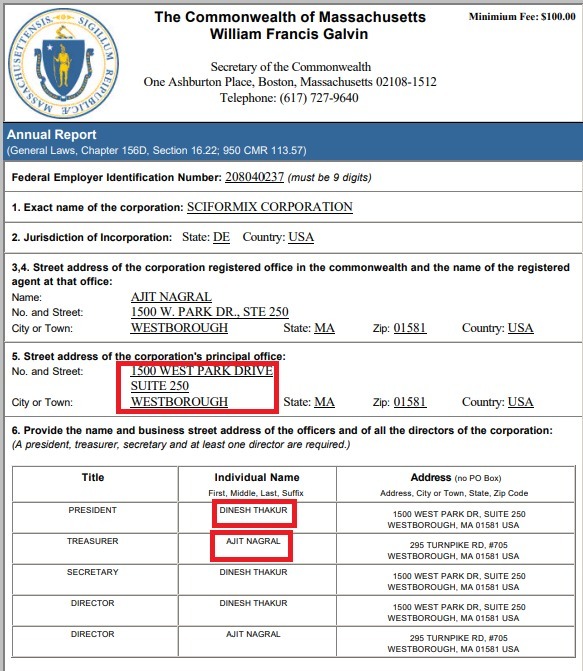
After leaving Ranbaxy, Thakur worked at Infosys as Vice President & Head, Healthcare & Life Sciences for a year from May 2005-April 2006. Then towards the end of 2006, on November 8, 2006, Sciformix Corporation, a Contract Research Organization (CRO) world, was co-founded by Dinesh Thakur and Ajit Nagral in the US and India. As per its website in 2007-08, Sciformix was a Knowledge Process Outsourcing (KPO) including providing high quality knowledge-based services for scientific data management, regulatory compliance and risk management, with the ultimate objective of improving quality of health care imparted to patients, worldwide [67].
Thakur took the designation of the President and CEO of Sciformix while his co-founder Ajit Nagral, became its chairman [68]. Sciformix Corporation is a scientific process organization (SPO), a global service provider for the biopharmaceutical, generic pharmaceutical, consumer product, medical device, and contract research industries formed with the vision of establishing a company to provide specialized science-based services to pharmaceutical organizations [69][70].
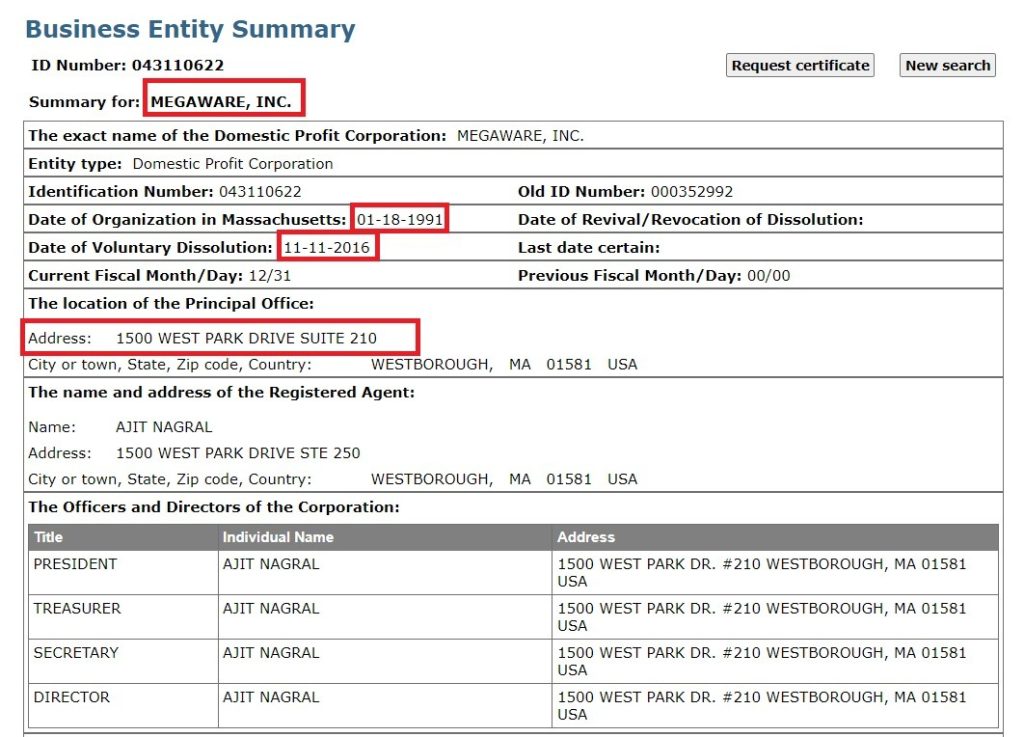
Before Sciformix, Ajit Nagral had founded and ran various companies under the Life Science and Technology domain including NuGenesis Technologies Corporation (1996). and Megaware Inc. (1991) in Massachusetts. Megaware Inc. is a software company that provides software products and services to the pharmaceutical, biotechnology, and other life science markets. It is worth noting that both Sciformix and Megaware were located at the same address in the US. Both Thakur and Nagral were together at the company till 2012.
Sciformix was a blooming business and had offices in India, the Philippines, and Europe [71][72].
Within a few months of its incorporation, Sciformix secured USD 3.3 million in Series A funding from Massachusetts-based Flybridge Capital Partners [73].
Ex-Ranbaxy Join Sciformix
Thakur was not alone, just after co-founding Sciformix, Thakur onboarded at least three of his associates from Ranbaxy.
One of them was Thakur’s immediate boss, Rajinder Kumar at Ranbaxy (Figure 1) [74]. He was Director of research and development from 2004-05 at Ranbaxy. Before Ranbaxy, he served as global head of psychiatry-clinical research and development at GlaxoSmithKline. He exited Ranbaxy in March 2005 and a year and a half later, he joined the newly formed Sciformix Corporations co-founded by Thakur and Nagral [75]. Rajinder Kumar was one of the key persons who supported Thakur in his quest of whistle-blowing against Ranbaxy with the help of the USA’s Food and Drug Administration (FDA).
Another person who quit Ranbaxy and joined Sciformix is Dr. Kathy Spreen. She joined just when Sciformix had embarked on its journey [76]. Before that, she had joined Ranbaxy’s US office as Executive Director of Clinical Medicine and Pharmacovigilance [77]. Before Ranbaxy, she worked at Wyeth and AstraZeneca. Apparently, it was Rajinder Kumar who apprised her of the data fraud at Ranbaxy. She went on to work with Thakur and Rajinder Kumar to expose Ranbaxy’s malpractices of data discrepancy for drug approval.
Roughly a year after Sciformix’s formation, Thakur’s old friend, Dinesh Kasthuril also joined the organization as its Director of Solutions and Consulting. During his stay, at the company, Dinesh Kasthuril went on to become the Director of Service and Delivery [78]. Before that, Dinesh Kasthuril worked at BMS (along with Thakur) as an Associate Research Scientist (Nov 1999-Jun 2003).
In India, Sciformix had offices in Andheri (Mumbai) & Pune. Members of Sciformix Corporation (Mumbai) included: Chitra Yeshwant Lele and Manish Vinayak [79}. Before joining Sciformix as Chief Scientific Officer in July 2007, Chitra worked at Pfizer as Executive Director of Development Operations India under Pfizer’s Global R&D between Jan 1998 to June 2009 [80].

Thakur- Kasthuril Leave Sciformix, Two Parallel Streams Began
Sciformix thrived, but years after being associated with Sciformix, as also documented in the book “Bottle of Lies: The Inside Story of The Generic Drug Boom” Thakur had some clashes with his co-founder Ajit Nagral in May 2012. It was also during the time Thakur had been making rounds to the FDA office over the Ranbaxy whistleblowing case. Following the internal differences, Dinesh Thakur finally parted ways with Sciformix in December 2012. The same was confirmed by Ajit Nagral during an event in Mumbai in 2018 [81] [82].

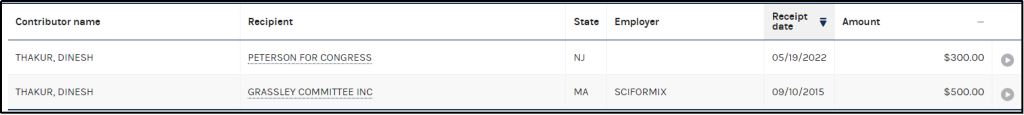
Note: Thakur resigned from Sciformix in 2012, and the same has been maintained by Nagral publicly. However, Thakur has been seen being employed by Sciformix post that period as available on the official government website of the US government. In 2015, Thakur contributed USD 500 to the Grassley Committee Inc. (Chuck Grassley (R)) and was shown as an employee of Sciformix [83].
And as Thakur parted ways with Sciformix, so did his friend Dinesh Kasthuril. Kastburil resigned from Sciformix in the same month as Thakur and went on to join a US-based Pharma Laboratory company Labcorp Corporation of America or LabCorp.
LabCorp primarily focussed on clinical laboratory testing and healthcare diagnostics, offering a wide range of services for physicians and healthcare providers [84]. Notably,
a Carolina-based healthcare company also owns Flybridge, which invested USD 3.3 million in Sciformix in 2008 [85].
Meanwhile, after receiving the whistle-blowing commission from the Ranbaxy episode, Thakur got into full-time ‘activism’ and as noted in Chapter 4, Thakur started his website. He got active on social media, and became a ‘public-health activist’.
Thakur’s Ventures Post Sciformix
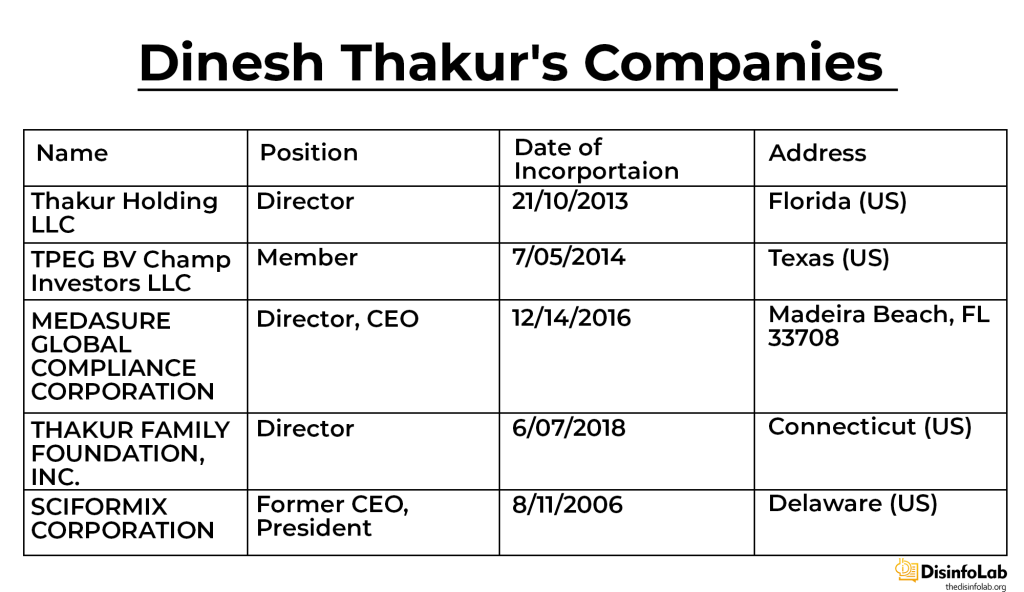
With the whistle-blowing money Thakur set up an investment company Thakur Holding LLC on October 21, 2013, in St. Petersburg, Florida. Thakur is still an active member.
Since 2014, Thakur has also been engaging with the US Congressional staff over the quality of made-in-India generic drugs imported to the US [86] with backing from academicians including Roger Bate, a fellow at the American Enterprise Institute (AEI) [87]. Incidentally, Roger Bate has been advocating for Thakur to get into a top job at the FDA.
On May 4, 2014, a domestic liability company named TPEG BV Champ Investors LLC was set up by its Texas-based parent company Trinity Private Equity Group (TPEG). Thakur has been part of the members’ list of the TPEG BV Champ Investors LLC to date. Other investors and partners include Bobby Altman Trust, Timothy Looney, Chandra Family Investments, Kotrappa Family Trust, etc. [88],
Sensing opportunity in contract research organization (CRO), on January 9, 2014, Thakur founded another company Medassure Global Compliance Corporation. According to its website (which is not available now), Medassure was a one-stop solution for all the supply chain compliance needs of the pharmaceutical, device, and consumer products industry [89]. As per its documents, Thakur has been a sole director and official. Although the company is a risk management consultancy services organization focused on the pharmaceutical supply chain, no details of its employees and officials are available apart from Thakur [90].

On July 6, 2018, Thakur founded a grant-making front named Thakur Family Foundation Inc., As per its website, Thakur Family Foundation is aimed at empowering and enabling citizens to improve their lives by financially supporting the cause of public health and promotion of civil liberties [91]. However, it is this ‘foundation’, set up with the Ranbaxy case fund, which is at the center of a major narrative against Indian pharma, starting with Covid in India.
Dinesh Kasthuril
As Thakur parted ways with Sciformix to set out on his new business and eventually activism venture. Meanwhile, across the globe, US-based Pharma LabCorp, a giant in clinical lab testing, was quietly plotting its ascent in the drug development domain. In 2014, it made a strategic move – acquiring Covance, another prominent CRO known for its clinical trial expertise [92].
Back in India, Kasthuril, post-Sciformix, had landed at LabCorp within a month of his resignation from Sciformix, likely sensing the company’s burgeoning ambitions.
Fast forward to 2014, LabCorp, fuelled by Covance, cemented its position as a drug development heavyweight. Dinesh Thakur, meanwhile, was pursuing independent ventures like Thakur Holding LLC and TPEG in the US, while also immersing himself in activism. While LabCorp consolidated its grip, Thakur grappled with the challenges of launching new businesses.
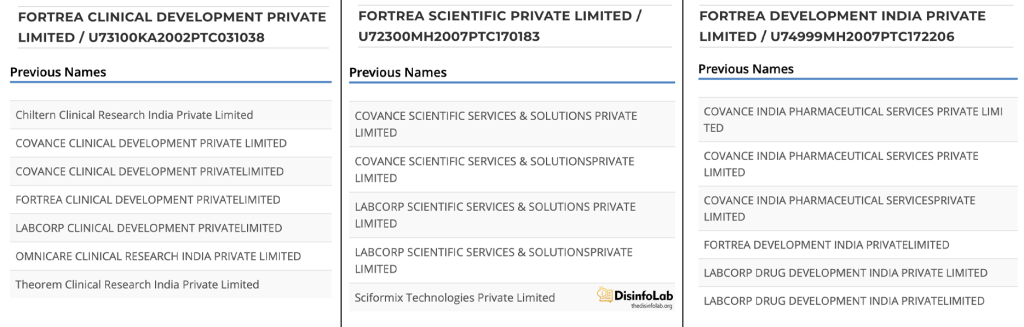
Intriguingly, in 2016, Thakur ventured back into the CRO space with Medassure, perhaps indicating a rekindled interest in his Sciformix roots. In a surprising turn of events, just two years later, in June 2018, LabCorp swooped in, acquiring Thakur’s old company – Sciformix. Therefore, at present, the website of Sciformix (www.sciformix.com) redirects to LabCorp.
Meanwhile, back in the corporate world, LabCorp made another strategic move in 2023 – a spin-off company to form Fortrea, an entity designed to house its CRO operations [93] [94]. With the formation of Fortrea, Kasthuril was appointed to the position of Global Head of Fortrea’s Post Approval Safety [95].
This meant that the India offices of both Sciformix and Covance, now under LabCorp’s umbrella, found themselves operating under the Fortrea banner.
The integration process unfolds, with India, a key center for both companies, playing a crucial role. Sciformix’s India offices merge with Covance’s to form the Indian arm of LabCorp and its new subsidiary, Fortrea, a CRO, which inherits Covance’s legacy business.

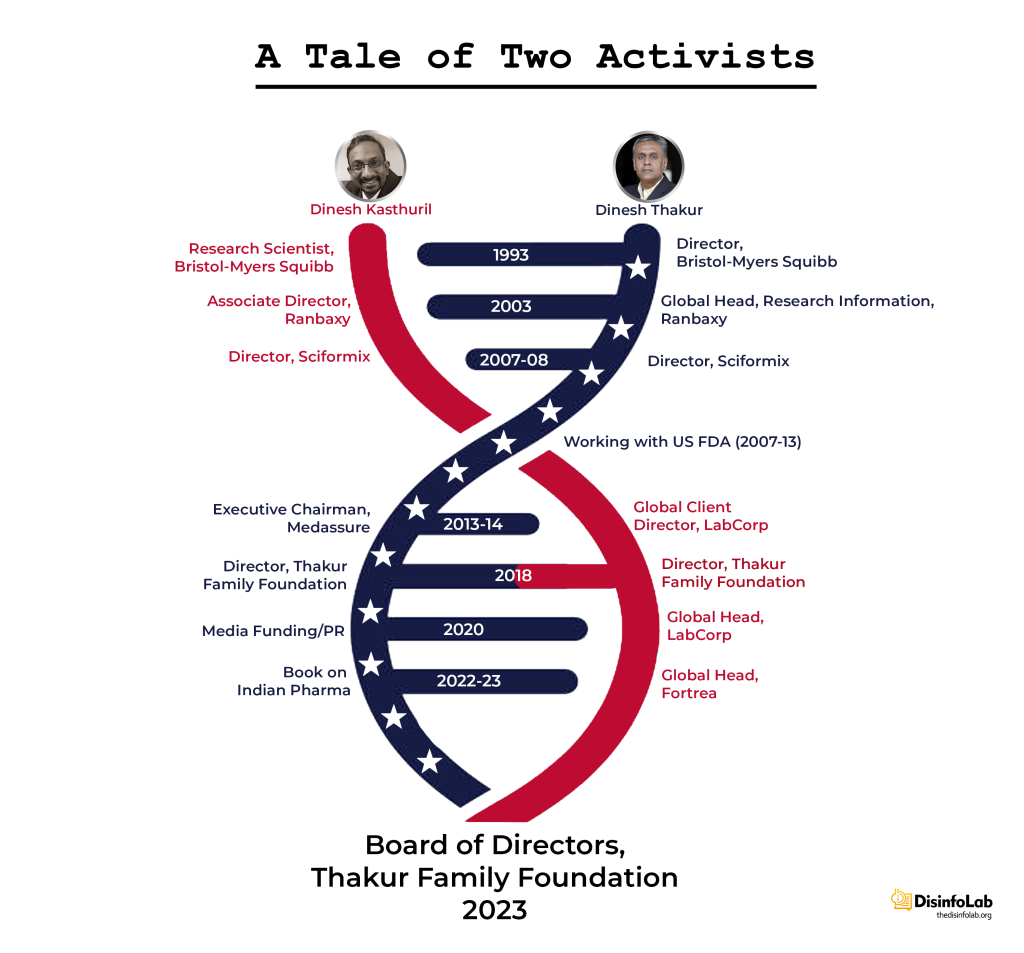
The Two Dinesh-es
Notably, a month after the acquisition, Thakur and Kasthuril, the men who once shared the helm at Sciformix, crossed paths again and established the Thakur Family Foundation. The Thakur Family Foundation soon shifted its focus around narrative building against the Indian pharma industry, particularly during the COVID-19 pandemic.
Today, Kasthuril remains associated with Fortrea, a significant player in the CRO landscape with its roots in both Covance and Sciformix. Dinesh Thakur, on the other hand, has fully dedicated himself to public health activism, becoming a vocal advocate through the Thakur Foundation.
From Sciformix to Fortrea and Thakur Family Foundation
Chapter 7: Making of A Messiah – Thakur Family Foundation
As per his self-eulogy, after the Ranbaxy episode in 2013, Thakur became a public health activist and started focusing on drug manufacturing overseas and identifying the problems, providing solutions to fix them, and also elevating the manufacturing standards of overseas facilities to those accepted by the FDA. [96]
Please note, that Thakur, a US citizen is only interested outside of the US, but NOT in the US where pharma sector is replete with malpractices. This is the greatness of all things American – from media to activists – they are devoted to correcting the wrongs only outside of US. And for some reasons, Indian origin Americans are at the forefront.
Post 2013, having set up a coffer for Namesake Foundation, Thakur have been trying to position himself as ‘health activist’ in India. To this end, he has been attempting various gymnastics – from Public Interest Litigations (PIL) to articles to Books.
Public Interest Litigation (PIL)
The first publicity stunt was in 2016, Dinesh Thakur and Prashant Reddy filed a Public Interest Litigation (PIL) before the Supreme Court of India against the Drug Controller General of India (DCGI) to raise awareness about alleged regulatory loopholes that led to the supply of substandard drugs. The PIL was filed based on a 123-page report titled ‘Fixing India’s broken drug regulatory framework’ [97]. The PIL was filed by four advocates Mr. Raju Ramachandran, Ms. Anita Shenoy, Prashant Reddy, and Ms Maitreyi Misra [98].
Some Facts About the Advocates:
Raju Ramachandran is a senior advocate at the Supreme Court of India and a former Additional Solicitor General. He served as an amicus curia for the Supreme Court of India in the 2002 Gujarat riots case and in the appeal of Ajmal Kasab in the 2008 Mumbai [99].
Ms Maitreyi Misra is a Research Associate at the National Law University and her past experience includes working as an International Law and Human Rights Fellow as part of which she worked with the Association for Civil Rights in Israel. She also worked as research officer under Mr. Anand Grover (Senior Advocate, Supreme Court of India), in his capacity as the UN Special Rapporteur on the right to health [100].
She is the founding member of Project 39A, a criminal justice initiative based out of the National Law University in Delhi [101]. Dinesh Thakur has made grants to both National Law University and Project 39A for several times under ‘civil liberties’ and ‘public health’ categories between 2021-22 [102].
However, Tirath Singh Thakur, then-Chief Justice of India (CJI) threw the PIL (within 15 minutes of hearing), stating that “How can you bring these academic issues? (It’s for) people who are affected. We will not entertain petitions from public interest activists who come here for publicity or whatever. Unless there is something very serious that affects someone, we will not entertain (it) [103],” SC not only refused to hear the case, it also ordered for the PIL to be withdrawn by Thakur, but gave him the liberty to file the petition to the government of India [104] [105].
As per a February 2016 article in the Economic Times (ET), Thakur had been considering plans to set up a patient advocacy group in India. For that, he had also been trying to reach out to some Public Relations firms in India. The article further stated that such an initiative will only go about when top Indian pharma companies would come under US FDA’s scanner for not complying with Good Manufacturing Practices (GMP) [106]. Thakur, however, denied those claims via an email to ET.
On May 7, 2018, Thakur filed a petition to the Government of India seeking the appointment of a commission of inquiry to investigate the ‘illegal approval’ of drugs by India’s Central Drug Standards Control Organization (CDSCO) [107]. This came a year after the Medical Council of India (MCI) asked medical practitioners to prescribe generic medicines to patients and write them down in clear and capital letters due to its affordable pricing of generic medicines in India [108]. In other words, Thakur was pushing back against prescription of generic medicines. Worth repeating that it was a fight over generic medicine between Ranbaxy and Pfizer.
Within three months Thakur started a new venture named Thakur Family Foundation (TFF) on July 6, 2018, in St. Petersburg, Florida, and subsequently onboards his friends Dinesh Kasthuril and Venkat Swaminathan, both of who have worked with Thakur at Bristol Myers Squibb (BMS) as well as at Ranbaxy before leaving the company in 2005. Thakur became its president and treasurer, while his other partner Prashant Reddy joined as its director [109].
And while Thakur and Kasthuril started TFF together, in March 2023, they also added Venkat Swaminathan as an officer at the TFF, replacing Prashant Reddy as Director [110]. The current board of directors of TFF comprises Dinesh Thakur, Venkat Swaminathan, and Dinesh Kasthuril [112].

Not much is available about the employees of TFF, but as per its LinkedIn page, TFF has six employees in and outside India. Its HR researcher Hem Raj is based in Mandi, Himachal Pradesh [112]. According to the LinkedIn listed employees of TFF, one of its employees, Rishi Thakur who is a Business analyst is based in Ghaziabad, India [113]. But upon looking further into TFF’s finances, we found that TFF’s investments and books are maintained by Merril Lynch, a Bank of America subsidiary. And one Rishi Thakur is also a financial solutions advisor at Merril Lynch [114].
TFF’s Research Assistant and Programme manager is a New Delhi-based Nazeen F. since August 2020. Nazreen graduated with an MA in Conflict Analysis and Peacebuilding, International Relations in 2015 from Jamia Millia Islamia, New Delhi. Before working at TFF, Nazreen worked as a research assistant at the New Delhi-based Centre for Equity Studies (CES).
| Centre For Equity Studies (CES): CES is a non-profit organization based in New Delhi, India. It was founded in 2014 by a group of ‘activists and scholars’ including NC Saxena, Ghanshyam Shah, Jean Dreze, Shekhar Singh, Usha Ramanathan, and Harsh Mander [115]. On June 20, 2023, the Foreign Contribution Regulation Act (FCRA) license of CES was cancelled for 180 days on charges of receiving foreign funds and releasing reports authored by non-FCRA organizations citing a 2020 report titled ‘Labouring Lives: Hunger and Despair Amid Lockdown’ which CES had co-curated alongside Delhi Research Group and Karwan-E-Mohabbat and was supported by Rosa Luxemburg Stiftung, a German foundation [116]. CES’ chairperson is the former IAS officer Harsh Mander in March 2020, was booked by the Delhi Police for inciting violence during the Citizenship Amendment Act (CAA) and making derogatory remarks against the Supreme Court of India [117] [118]. A year later, a case was registered under the Juvenile Justice (Care and Protection of Children) Act against two NGOs Umeed Aman Ghar (Aman Biradari Trust) and Khushi Rainbow Home, (both owned by Mander’s organization Centre for Equity Studies) over child sexual abuse [119]. Mander is also on the board of Quill Foundation, which is an autonomous institution engaging in research and advocacy in India and promotes a fabricated ‘DOTO’ database to staple the Islamophobia tag on India. Mander’s footprints can be seen in many anti-India campaigning organizations. He was the Chairperson of the Advisory Board of the Human Rights Initiative of the Open Society Foundations from 2020-22, and Member from 2018-20 [120]. He was also one of the advisors of ISI puppet Muhammad Ayyub Thakur’s The Justice Foundation (as noted in the previous Disinfolab dossier on Kashmir in 2021) in 2007. |
Crusade Against Generic Drug
As noted in previous sections, much of the Thakur’s career especially his stint with BMS, followed by Ranbaxy and whistleblowing has been accounted in the book series Bottle of Lies. The author of the book series is a US-based journalist and author, Katherine Eban Finkelstein.
She is currently special correspondent of the portal Vanity Fair since April 2020. Previously she has worked as a contributor and reporter for numerous portals including New York Magazine, The Nation, ABC News, New York Times, Fortune Magazine, and SELF Magazine [121].
In 2013, Katherine Eban received USD 50K funding from a New York-based philanthropic organization, Alfred P. Sloan Foundation. The money was granted ‘to write a book on the dangers of America’s use and manufacturing of generic drugs.’ [122] Incidentally, she goes on to write two book under the series ‘Bottle of Lies’ to delve into the dark side of the generic drug industry.
Six years after receiving the money, in 2019, Katherine released her two-book series under Bottle of Lies highlighting the Ranbaxy whistleblowing incident. Her first book of the series ‘Bottle of Lies: the Inside Story of the Generic Drug Boom’ was released on May 13, 2019.
The centre of the book series is Ranbaxy, its history, people, culture, and data falsification incident and whistleblowing by Dinesh Thakur, leading to its ultimate downfall and later pleading guilty to the charges (in 2013) levied by the FDA.
Her second book of the series ‘Bottle of Lies: Ranbaxy and the Dark Side of Indian Pharma’ was released in July 2019. In this book, Eban dives into the details of the institutional malpractices followed by some Indian pharmaceutical companies, keeping the story of Ranbaxy’s rise and downfall again at the center.
Chapter 8: Its Iraq Again- Thakur, Bloomberg & Valisure
To what extent the cartel could go to damage Indian pharma?
In July 2023, Bloomberg News commissioned a test of medication syrups that were made in India. For the test, Bloomberg purchased 33 samples of the made-in-India syrups from pharmacies in Cambodia, Georgia, Ghana, India, Iraq, and Kenya in March 2023. Out of those one of the syrups “Cold Out”, a cold-curing medication was which was made in India and sold in Iraq was allegedly found to be contaminated with dangerous toxic chemicals.
According to the test findings, the syrup contained 21 times the acceptable limit of ethylene glycol, a compound lethal to humans in small amounts. After the testing, Bloomberg submitted the findings to Indian and Iraqi authorities, as well as to the World Health Organization (WHO) [123].
The allegedly tainted cold syrup was manufactured by a Chennai-based Fourrts (India) Pvt. Ltd that had in turn sub-contracted the medication’s manufacturing to a Puducherry-based Sharun Pharmaceuticals Private Limited. Following Bloomberg’s inquiry, Fourrts Pvt Ltd also tested the allegedly tainted samples. However, the company did not find any tainted substance in that. Following this incident, the Iraqi government recalled all the tainted cold medicine imported from India [124] [125].
It is important to note that the test commissioned by Bloomberg was conducted by a Connecticut-based lab Valisure LLC. As per its website, Valisure is an independent laboratory and partner for quality that works to increase transparency and quality assurance throughout the healthcare industry and operates independently of the FDA [126]. Valisure was co-founded in 2015 by two Yale University students and friends, David Y Light and Adam Clark-Joseph [127].
How reliable is Valisure and its testing?
Valisure hit the headlines in 2019 when it conducted a study on Zantac (once a top-selling heartburn medication) and its generic equivalents; and claimed to unearth that the medication contained a high concentration of N-Nitrosodimethylamine (NDMA) also thought to be probable carcinogen that could cause cancer [128] [129]. Following that, Valisure filed a citizen petition with the FDA, asking the agency to recall all products containing ranitidine. Alarmed by the ‘revelation’, thousands of cancer patients filed a case against the company.
However, on December 6, 2022, a US federal court dismissed lawsuits in multidistrict litigation in Florida. In the 341-page ruling Judge Robin Rosenberg found that the plaintiff’s experts (Valisure) did not resort to reliable methods to conclude that Zantac was linked to causing Cancer, and stated that the Plaintiff’s experts “systemically utilized unreliable methodologies with a lack of documentation on how experiments were conducted, a lack of substantiation for analytical leaps, a lack of statistically significant data and a lack of internally consistent, objective, science-based standards for the even-handed evaluation of data,” [130]
In its test, Valisure claimed that NDMA content in Zantac exceeded 3,000,000 nanograms (ng) over the daily prescribed limit of NDMA by the FDA i.e., 96 nanograms. And in the 341-page ruling, it was also revealed that Valisure heated the ranitidine to 266 degrees Fahrenheit way above the 98 degrees Fahrenheit temperature of the human body in order to artificially achieve the result of 3,000,000 ng. Upon testing the ranitidine at 98 degrees Fahrenheit, Valisure did not find any NDMA [131] [132]. However, due to its testing methods against Zantac, invited thousands of lawsuits including over 2000 private damages and over 70,000 claims registered by consumers of the remedy in litigation [133].
In another instance, on December 5, 2022, the FDA sent an 8-page letter to Valisure highlighting its concerns over Valisure’s failure to comply with Current Good Manufacturing Practices (CGMP) prescribed by the FDA. The letter was in follow-up to the inspection of Valisure’s drug testing laboratory by FDA officials from May 26 to July 2021 [134]. The FDA found that Valisure’s methods were not validated, their equipment was not qualified, and they lacked control over their computer systems. The FDA also cited concerns that Valisure’s testing could be used for regulatory purposes, even though Valisure claims it is only for informational purposes [135].
In the letter, the FDA also noted that Valisure LLC also had a subsidiary ValisureRx, a licensed pharmacy and whole drug distributor, and that Valisure LLC, while owning ValisureRx, violated the drug supply chain security requirements. Valisure LLC made money by selling only certified generics. ValisureRx was however sold to Medly Health in April 2021 [136]. Lastly, the FDA also concluded that Valisure never filed the annual report with the FDA as required by the regulatory body [137].
Thakur, Eban, and Valisure
Notably, around the same time that Bloomberg commissioned the test, Thakur Family Foundation (TFF) issued an ‘Awarded’ to Valisure to ‘PERFORM A QUALITY RISK STUDY ON THE DEPARTMENT OF DEFENSE PHARMACEUTICAL’. However, no further details nor the amount awarded to Valisure was disclosed at the time of making this report.

The connection goes deeper. Both Thakur and Katherine Eban seem to have endorsed Valisure on numerous occasions in the past [139] [140] [141]. On September 9, 2020, Valisure organized a webinar and invited Katherine Eban, along with Joe Graedon, founder, editor, and podcast host of The People’s Pharmacy to discuss the pervasive issues with overseas drug manufacturing, fraud, and limitations of government oversight and how all of it puts American consumers’ lives at risk [142].
Thakur continues to endorse Valisure’s study on Zantac which was discredited in the court and while the company also lost credibility following the FDA letter questioning their methods of testing [143]. More importantly, as noted earlier in this chapter, the Thakur Family Foundation granted them funds for conducting a study in the field of defense pharmaceutical.
Agreement with the US Department of Defense
On August 8, 2023, Valisure LLC entered into a Cooperative Research and Development Agreement (CRADA) with the US Department of Defense (DoD) to jointly conduct a pilot study to assess the quality of drugs supplied to the DoD. The study was slated in response to concerns about the quality and safety of ‘generic drugs’, many of which are manufactured in India and China [144]. The drugs were to be tested for a variety of factors, including potency, purity, and contamination to hold the manufacturers accountable based on results [145].
However, if one were to see Valisure history, the DoD decision would seem perplexing.
| BUT: Bloomberg News commissioned the test of syrups by Valisure, which had already been exposed to have faked such tests. Coincidentally, after few days, Valisure signed the agreement with US DoD; AND received ‘awards’ from Thakur Foundation. Unrelated, but Michael Bloomberg the co-founder and CEO of the Bloomberg is the current Chair of the Defence Innovation Board (DIB) [146], and provides recommendations to the Secretary of Defense and other senior DoD leaders on emerging technologies and innovative approaches that the DoD should adopt to ensure U.S. technological and military dominance [147]. |
Some Questions
What triggered Bloomberg to conduct tests on Indian medicine? Does a news media usually engage in such activities? Was it only for Indian medicine or others as well? What were the reasons for selecting Valisure, what were the criteria? Who funded for the logistics? Did Bloomberg get the process and outcome vetted by a third party?
Amongst several countries the sample were picked up from, only one sample was found to be contaminated. Did Bloomberg eliminate the possibilities of local contamination and/ or fake manufacturing? What was the process of ‘buying’ these medicines, and how was it ensured the integrity of the process?
Part III: Controlling The Narrative Eco-system
Chapter 9: Thakur’s Grants to Indian Media
Without the support of a well-oiled machinery, it is virtually impossible to push any targeted narrative. The same is case with Thakur Family Foundation or TFF fundings – while TFF sponsored several media and journalists to do reporting, only a handful were relied upon to push a particular story-line, such as The Wire. With the same source of funding, many other portals such as Alt News and Caravan have done useful reporting. We will return to such orchestrated reporting in a while.
Starting in 2019, TFF started engaging with think tanks, media houses, and independent journalists to promote its agenda, though it is likely that many of the recipients would have been unaware of the design. TFF made handsome contributions in various fields including the Public Health sector etc. TFF set to create an ecosystem of journalists and media portals to push the narratives it wants through funding.
TFF grants had the following criteria for applicants’ eligibility [148]:
- The applicants needed to write a 15,000-word investigative report or a 25-minute documentary published by reputable media houses.
- The grants were for four months beginning September 1, 2020, and the assignment would have to be the applicant’s full-time work.
- The grant pays INR 250,000 for the duration of the assignment;
- INR 50,000 per month in compensation and up to INR 50,000 in substantiated reporting expenses.
- One of the topics: ‘How are Ayurvedic products developed, tested, approved by regulatory authorities, marketed, and manufactured under the current regulatory structure?’
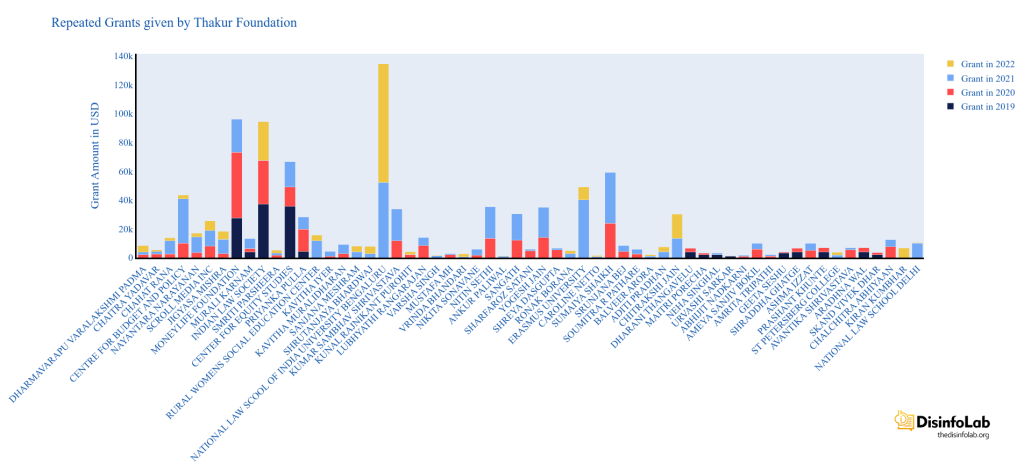
In 2020-21 when COVID-19 was at its peak in India, several media portals News Laundry, Scroll, Article14, The Wire, and their journalists including Priyanka Pulla, Banjot Kaur, Shreya Dasgupta, Kumar Shambhav, and Nitin Sethi, among others were engaged by Thakur Family Foundation (TFF). Some of them have been engaged by the TFF even before COVID-19 i.e., since 2018-19 which will be elaborated in later sections in this report.
DIGIPUB News India Foundation
Notably, these media portals are part of the DIGIPUB News India Foundation. The Chairperson of DIGIPUB is Dhanya Rajendran of The News Minute and the General Secretaries of DIGIPUB are Ritu Kapur of The Quint and Abhinandan Sekhri of NewsLaundry. One of the members of the Internal Committee of DIGIPUB is Bezwada Wilson, activist and National Convenor of the Safai Karmachari Andolan [149]. Safai Karmachari Andolan has also been a beneficiary of the funding by the Thakur Foundation, which granted USD 18,353 & USD 27,275 in 2019 and 2020 respectively. Both times the funding was granted for the purpose of ‘civil liberties’.


Scroll. In
India’s Scroll media received USD 8,000 and USD 10,810 in 2020 and 2021, respectively, from the Thakur Family Foundation. Scroll received funds under the public health category for publishing as many as 17 articles highlighting the worsening’ situation in different parts of India during COVID-19.

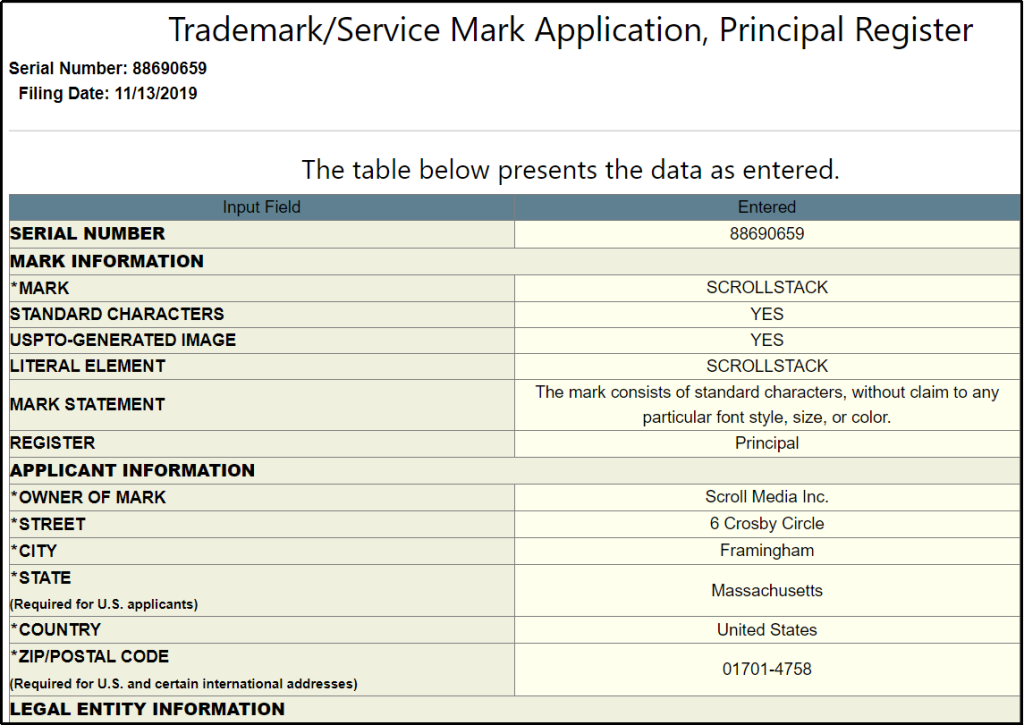
For articles written during the first COVID-19 wave in 2020, Scroll Media Inc., the money was wired to the address 6 Crosby Circle Framingham, Massachusetts, 017014758, United States. The company registered at the address is Scroll Media Inc. as Scrollstack, according to the trademark/ service mark application filed in 2019 [150].
News Laundry
Another media portal engaged by TFF was NewsLaundry. The portal was contracted to publish at least seven stories on its platform highlighting the situation in India due to COVID-19 between April 23- June 10, 2020.
As per the 2020 financial filings of the Thakur Family Foundation, they paid USD 11,188 to a New Delhi-based Toque Content Solutions which is registered by Newslaundry’s cofounders Abhinandan Sekhri and Madhu Trehan [151]. The previous directors were also co-founders of Newslaundry, Prashant Sareen and Roopak Kapoor [152].


Incidentally, Toque Content Solutions was registered in June 2018, and at some places it has different address than News laundry while on some company directories, it shares the same address as Newslaundry [153] [154].
For seven articles (including video series), NL was paid INR 8,37,800 or USD 11,188 by the Thakur Family Foundation. The grant partnership was coordinated by NL’s partnership lead at that time, Shubh Soni, who worked at NL for a year between March 2020 and April 2021.
Shubh Soni has previously worked at the Observer Research Foundation (ORF) as a research assistant, research fellow, and program coordinator between May 2015 and 2017. He then worked as an additional private secretary at the Ministry of Housing and Urban Affairs, Government of India (GoI) between Nov 2017 and 2019. Furthermore, he worked as Campaign Coordinator & Public Relations lead at Bharatiya Janata Party (BJP) from Amritsar constituency for two months between April 2019-May 2019. Shubh is currently the head of forums & partnerships at ORF since April 2021 [155].
It is not clear whether the funding had a bearing on NL’s articles, which defended Thakur and Prashant Reddy, when the duo received a notice from India’s Central Drugs Standard Control Organization (CDSCO). However, NL did not provide a disclaimer (even if unrelated) that it had received funding from TFF, while defending him.
Saamarasya Media (Article 14)
Saamarasya Media is a Limited Liability Company (LLC) based in Karnataka. The company was registered on July 7, 2020, by Javed Mustafa Ansari, Samar Padmakar Halarnkar, and Betwa Sharma. The company owns one of the newly established media portals Article 14. [156]
Notably, within a year of its formation, Saamarasya Media was engaged by TFF (in 2021) to publish articles under the public health and civil liberties category. Thakur Family Foundation paid USD 1,244 to the media company under the “civil liberties” categories. The portals covered six articles under the contract [157] [158] [159] [160]. In terms of economy, Saamarasya Media/Article 14 came cheapest for TFF.
Between 2020 and 2022, apart from the direct funding to the portal, Article 14 has published a total of 23 articles by journalists individually funded by TFF, both in the public health (9) and civil liberties (20) categories combined. The journalists, who have been engaged by TFF under public health grants include Kavitha Iyer, Nitin Sethi, Kumar Shambhav Shrivastava, Kunal Purohit, Bhanupriya Rao, Mridula Chari, and Saurav Das.
An anomaly: Saurav Das received USD 3,381 in 2020 for writing on public health. However, he has not written any TFF-funded article in the public health category. Instead, he has written just one story for Article 14 on Pegasus. This is contrary to all the journalists who have received funding from TFF for writing on the public health sector and have stuck to the category in their articles.
Alt News
Thakur Family Foundation also engaged Alt News journalist and doctor Sharfaroz Satani who wrote as many as eight articles during the first wave of COVID-19 in 2020. As per his bio, Dr. Sharfaroz Satani is a science writer and a drug and safety physician who also worked as a manager at OrciMed Life Sciences Private Limited since January 2023. He has previously worked at GSS Pharma (April 2020-January 2023) [161].
Satani’s articles ranged from questioning home remedies to cure COVID-19 [162] [163] to busting fake advisory news to curb COVID-19 [164]. Some of his articles also claimed that “AYUSH Kwath or Kadha cannot ‘boost’ immunity to fight against COVID-19 [165]” And also busting misinformation around the virus [166]. For that, Dr. Satani was paid USD 5907 between 2020-21.
Alt News Science’s founding editor (from 2017-21), Dr. Sumaiya Shaikh was also hired by TFF and received handsome compensation. Her works featured 10 articles including ones co-authored with Dr. Satani [167] [168] [169] [170] [171] [172] [173]. For her work, Dr. Sumaiya Shaikh received USD 23,740 & USD 35,260 from TFF in 2021 & 2022 respectively. The article helped dispel some prevailing rumours and disinformation, though some on traditional Kwath/ Kadha bordered on being pedantic.
| Note: As noted in this section, TFF had started engaging journalists, NGOs, and institutions since 2019. During that year, the foundation made transfer of the funds directly to the bank accounts of journalists; and individuals it engaged with. However, from 2020 onwards, TFF hired financial service institutions to send money to India to individuals/ journalists/ activists it engaged with. As per the Section 3(1) of FCRA, 2010, foreign contribution cannot be accepted by any: (a) a candidate for election; (b) correspondent, columnist, cartoonist, editor, owner, printer or publisher of a registered newspaper; (c) Judge, government servant or employee of any Corporation or any other body controlled on owned by the Government; (d) member of any legislature; (e) political party or office bearer thereof; [174] In February 2020, Dinesh Thakur filed a plea to the Government of India (GoI), arguing that overseas citizens be allowed to donate to religious and charitable institutions in India without the need for prior permission under Foreign Contribution Regulation Act, 2010 (FCRA) [175]. Incidentally, around the time, Thakur started funding Indian news portals, NGOs, and civil groups in India and until that time, as noted, TFF had been sending money to individuals in India directly in their personal bank accounts. |
Awards Granted by TFF to Journalists in India
It is worth highlighting that the fact of funding from Thakur doesn’t ipso-facto establishes any malpractice on part of the news portals, as long as the route of funding was legit and the adequate disclaimers were provided. In fact, some of the news portals did do some much-needed reporting about the aspects of covid that was both timely and needed. Many stories by The Caravan, News Laundry, and some others were qualitatively good journalism, and so were some of the useful fact-checks by Alt-News.
However, some of the news portals were engaged in blatant propaganda (we detail later), and in many cases, even the otherwise seemingly legit articles did not follow good journalistic practices. Many journalists, knowingly or unknowingly, while working on the articles funded by TFF, quoted opinions by ‘experts’, who themselves were either funded or affiliated with Thakur/ TFF. And even if the article provided a disclaimer, it did not provide details about the individuals’ association. And while this could have been excusable as an innocuous mistake, the fact that ALL of such experts had almost invariably one type of view, is too much of a coincidence.
Articles/ Reports commissioned by TFF Since 2020
Chapter 10: Narrative Control During COVID-19: an ‘Experts’ Case Study
Now that Thakur’s love for India became clear, it was important to see what narratives were peddled through the sponsored articles. On August 15, 2020, in the midst of pandemic and lockdown in India, Thakur Foundation launched Investigative Reporting Grants in Public Health in India [176]. On the face of it, the objective behind the grant was to raise awareness of India’s public health and influence policy by bringing India’s public health issues into the mainstream. As such, there was nothing wrong in TFF funding India media/ journalist, and they in turn doing a critical reporting. After all, this is for what independent media exist in India. However, there are certain basic journalistic practices one is supposed to follow. These practices, followed globally, are designed for this particular reason – to prevent news media from becoming propaganda outlets. One of the core principles, especially for sponsored articles, is adequate disclaimer and transparency. While some media did put such disclaimers, others didn’t bother.
This was where the seeding for the narrative building was beginning to undermine India’s fight against the virus. Thakur’s tirade against India has been focussed on denting Indian pharma’s image in general, and on Indian generic medicine and Indian vaccine in particular. And not surprisingly, the articles through journalist sponsored by TFF happened to be aligned to same narrative, particularly in platforms such as The Wire, and journalist like Priyanka Pulla among others. What was even more telling that several Thakur funded media articles quoted ‘independent experts’, who in turn were also funded/ affiliated with Thakur. And almost none of the article provided any disclaimer about their affiliations and conflict of interest. Coincidentally, all these ‘experts’ opinions have been consistently in one direction, aligning with Thakur’s.
Below is a table of some of such ‘reporting’ by some of the TFF supported news media/ articles.
| No | Article | Portal/ Author | Experts Quoted with linkages to Thakur/Pfizer/USA |
| 1 | COVID-19 Vaccine: What We Stand to Lose If ICMR Gets Its ‘Express’ Clinical Trial | The Wire Science- By Priyanka Pulla | Anant Bhan, a Bioethicist. He is an ex-advisory board member and ex-board of directors of TFF; and also serves as the advisory board member of DRISHTi, a Thakur funded initiative. Shahid Jameel: Virologist and CEO at the Welcome Trust-DBT India Alliance. He serves as the advisory board member of Thakur Foundation-funded DRISHTi. He was also signatory to a petition filed by Thakur to the Minister for Health. Incidentally, Jameel has previously been a keynote speaker at an event co-organized by HfHR & IAMC, both Pak Jamaat affiliated fronts. Urmila Thatte: Speaker at “Suno India” podcasts held on 13 March 2021, the episode funded The TFF. Jacob John/ CMC: Professor of community medicine at the Christian Medical College (CMC) Vellore. Incidentally, he is repeatedly quoted in TFF funded articles in other portals including Newslaundry, Scroll, The Wire (Science), and Science.org. Quite a coincidence, that so many different journalists from across dozens of media would find a set of 3-4 people from CMC Vellore as experts – in country with thousands of hospitals and hundreds of thousands of medical practitioners. What are the odds? |
| 2 | How Covaxin became a victim of vaccine triumphalism The report is in turn based on another article, funded by the Thakur Foundation, authored by Priyanka Pulla. | Live Mint By Priyanka Pulla | Gagandeep Kang: Microbiologist at CMC Vellore and Professor of Microbiology, at the WellCome Trust Research Laboratory, Division of Gastrointestinal Sciences at CMC. She is one of the advisory board members of DRISHTi. She is also connected to The Lancet on its “Covid-19 Commission”. Satyajit Rath: He is a faculty at the DRISHTi project funded by TFF. T Jacob John or Jacob John (noted above) |
| 3 | Scientists criticize rushed approval of Indian COVID-19 vaccine without efficacy data | Science.orgBy Priyanka Pulla | Gagandeep Kang |
| 4 | Serum Institute Fracas Exposes Loose Ends of India’s Clinical Trial Machinery | The Wire- By Shreya Dasgupta | Jacob John Anant Bhan |
| 5 | India needs more transparency in its COVID-19 vaccine trials, critics say | Science.orgBy Shreya Dasgupta | Amar Jeshai Anant Bhan |
| 6 | How the Modi government’sfailure to act led to India’sCOVID-19 catastrophe | Thakur Foundation website- By Chahat Rana | Dr Vikram Patel: Advisory board of the Thakur Foundation-funded project DRISHTi. |
| 7 | ‘It’s unscientific’: Doctors entrusted with Covid-19 vaccine trial slam ICMR’s August 15 deadline | Scroll-byArunabh Saikia | Vasantha Muthuswamy: She is a faculty at the Thakur Foundation funded-project DRISHTi. Amar Jesani: One of the signatories of a petition filed by Dinesh Thakur in August 2020 to Health Ministry. |
| 8 | Serum Institute’s trial has run into a controversy. Will this erode public trust in a vaccine? | Scroll- ByArunabh Saikia | Anant Bhan |
| 9 | Modis vaccine policy is a mistake other countries avoided | Caravan Nayantara Narayanan & Chahat Rana | Dr. Indranil Mukhopadhyay is a professor at School of Government and Public Policy at OP Jindal University (Haryana). The article was funded by TFF, and separately, Dr. Mukhopadhyay also received funding from TFF. |
(DRISHTi is a project of a Bhopal-based organization Sangath, which the Thakur Foundation funds. Sangath, a public mental health NGO, has received a total funding of USD 30,358 from the Thakur Foundation between 2020-2021.)
These are only SOME of the articles we analysed, with their ‘experts’. Other articles too have followed the same lead, getting funds from TFF and taking Quotes from TFF funded/ affiliated experts. These articles are not written for their sake alone. They have multiple usage. For example, an article funded by TFF in Scroll titled How India failed those who were harmed by the Covid-19 vaccine [177] by Tabassum Barnagarwala has quoted Dr. Jacob Puliyel, a former member of the National Technical Advisory Group on Immunisation, or NTAGI. What the article doesn’t mention is, Jacob Puliyel had filed a petition on June 25, 2021 in the Supreme Court against Union of India, which among other things questioned the “usage of made-in-India COVID vaccine, Covaxin which had been rejected by several countries.” Mr. Puliyel was represented by the eminent lawyer Prashant Bhushan [178]. The petition refers to many articles including an article titled “Explained: Is the Data From Covaxin Trial’s Bhopal Site Tainted?” published on February 9, 2021, by Priyanka Pulla on The Quint. The article was funded by the Thakur Family Foundation!
The Supreme Court refused to entertain the PIL.
But this was not an isolated case. Thakur himself has filed PILs, and as a base, he has used article published under his funding, quoting exerts who have also been funded/ affiliated with Thakur.
Chapter 11: Funding to Fame
To understand the gaining popularity of Dinesh Thakur, one must understand his journey after his whistleblowing incident which has been explained in Chapters 5, 6, & 7. However, since 2020, with the onset of COVID-19, Thakur’s popularity has jumped manifold as shown in the following graph and explained by corresponding events:
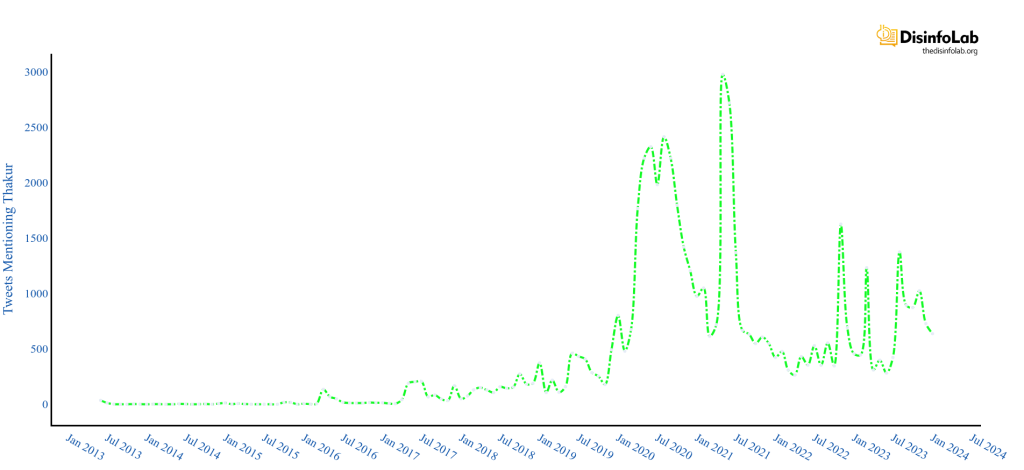
The first small spike we see in January 2019, corresponds with the time of the formation of the Thakur Family Foundation. It was in 2019 when TFF started making grants for public health and civil liberties subjects in India. It was also the time when he was featured as one of the central figures in Katherine Eban’s books in 2019.
But the biggest rise in his popularity graph took place in 2020 when the COVID-19 pandemic struck India, starting in February. Thakur Family Foundation funded several news portals in India to cover of the pandemic in India and how India dealt with the pandemic, followed by the coverage of the indigenous vaccines in the coming months. His grant-making further increased in 2021 during the 2nd wave of COVID-19 in India.
Not surprisingly, a large chunk of the articles targeted health ministry, b the Indian Council of Medical Research (ICMR), AYUSH Ministry, UNANI, CDSCO, and Central Drugs Standard Control Organisation (CDSCO) affiliated with India’s Ministry of Health.
Articles funded by TFF correspond with the spike i.e., his popularity on social media between 2020-21. We will analyse some of the article to show their pattern, likely orchestrated by Thakur and company.
Targeting CDSCO for Generic Drug Approvals
By 2022, Thakur had funded so many Indian news portals, that when Gambia case was reported, media portals suddenly rushed to Dinesh Thakur as their ‘go to’ quote person. They introduced Thakur as a ‘Public Health Activist’ while promoting the book “The Truth Pill: The Myth of Drug Regulation in India” which launched on October 10, 2022, rather interesting coincidence.
The first of many such reports, promoting the book and its authors was by The Wire on October 6, 2022, and its headline read, “Maiden Pharma, the Company Whose Syrup Killed Gambian Children, Is a Habitual Offender” by Banjot Kaur Bhatia [179]. The article, besides writing about Maiden Pharmaceuticals mentioned Thakur in the backdrop. Notably, Banjot Kaur Bhatia was among many journalists engaged by Dinesh Thakur’s grant-making foundation in 2020 [180].
The book talks about India’s generic drug industry and targets India’s Central Drugs Standard Control Organisation (CDSCO), India’s national regulatory body for cosmetics, pharmaceuticals, and medical devices. Thakur’s 454-page book talks about the CDSCO over 280 times.
In an interview with India Today dated October 11, 2022, Thakur and Reddy accused the Drugs Controller General of India (DCGI) of issuing a COPP (Certificate of Pharmaceutical product) to Maiden Pharmaceuticals whose cough syrups were sent to Gambia. The duo also accused the CDSCO of allowing the issuance of CoPP to the company [181]. Worth noting the role of Indian medicine in Gambia tragedy was never established.
One of the Indian newspapers, Times of India published an article on Jan 2, 2023 by Lata Mishra titled “‘Doctors don’t trust all medicine made in India’”. The article quoted the same two gentlemen mentioned above [182]. Another article by TOI again, on Jan 1st, 2023 “how drugmaker’s gamed the regulatory system” [183].
On Mar 23, 2023 Thakur and Reddy co-wrote OpEd, support of which was provided, in part, by the Bill and Melinda Gates Foundation [184], which seeks a worldwide medicines treaty to avoid further tragedies. The OpEd mentioned “… look into the cases of the last 6 months”. It further adds, “The response from India’s government, which generally boasts of the country being the pharmacy to the world, has oscillated between blaming the affected countries, conspiracy theories, and typical non-action” [185].
Chapter 12: Crescendo – Thakur meets Varadarajan
What happens when one India loving American meets another Indian loving American- both of Indian origin?
– an Outright Propaganda
From declaring that India’s situation is worse than Pakistan’s during the Covid-19 pandemic, curating a flurry of articles that include criticizing indigenous vaccines, to giving a platform to a bunch of TFF-funded articles during the pandemic, there is one portal that has been at the frontline of it all- The Wire. Most of The Wire’s article during this time on health and vaccine related issues are either funded by Thakur or quoting Thakur-connected people – and coincidentally, all are overwhelming negative.
But The Wire and Thakur’s links go back in time, starting 2015-16 when the Wire was just founded. The Wire was officially founded on May 11, 2015. [186] And Thakur has been writing on the portal since 2015 [187].
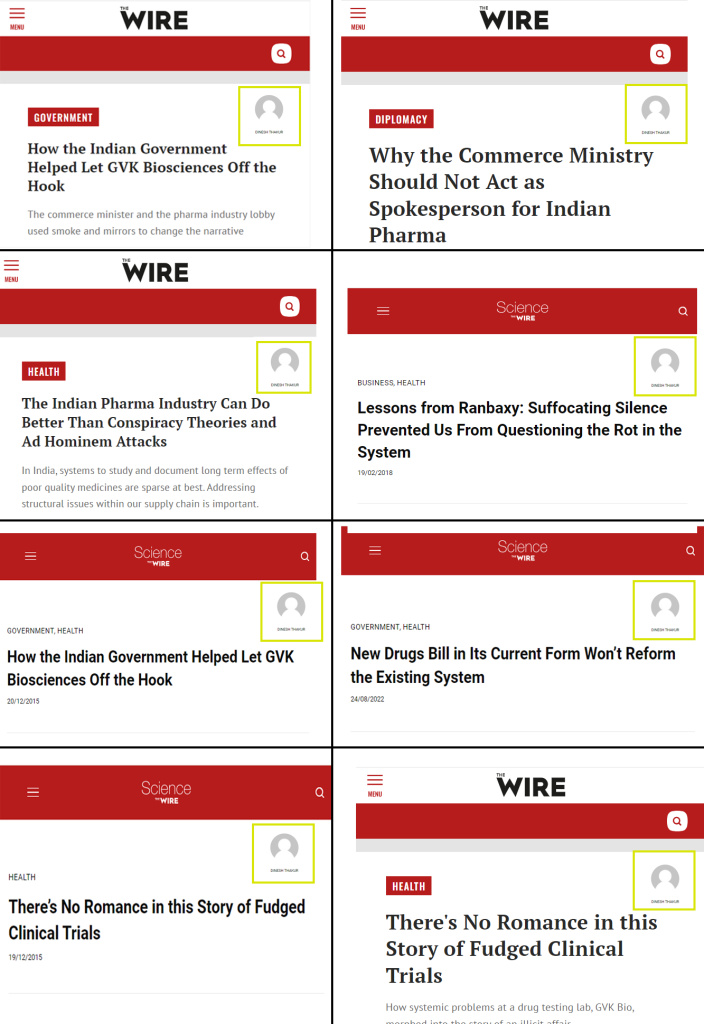
The Wire and Thakur’s roads have converged on various occasions. The Wire’s co-founder and editor, Siddharth Varadarajan was the advisory board member of Project 39A, funded by Thakur Family Foundation [188]. In February 2020, Siddharth Varadarajan interviewed the Bottle of Lies author Katherine Eban to discuss the subject “How Drug Makers Are Compromising the Lives of People.” [189]

Not only Thakur had written for The Wire, he happened to find the portal most suitable for churning his narrative during COVID-19. One of them being, targeting India’s indigenous COVID-19 vaccines including COVAXIN.
The Wire propaganda during COVID-19 years hit a crescendo, which led to a INR 100-crore defamation suit filed by Bharat Biotech, the manufacturer of an indigenous vaccine COVAXIN, against The Wire (elaborated in subsequent sections) [190]. The Wire published as many as 14 articles that were deemed critical of the company and the portal was directed to take down all the articles by the Telangana court hearing the case. Notably, one of the articles that defamed Bharat Biotech was written by Priyanka Pulla and was funded by Thakur Family Foundation [191].
And while The Wire was at its best of the forms in targeting indigenous COVAXIN among other propaganda pieces during COVID-19 time in India, personally, Dinesh Thakur has also been a vocal critic of the indigenous vaccine COVAXIN.
On June 6, 2021, The Wire’s Arfa Khanum Sherwani interviewed Dinesh Thakur to speak on vaccine approval process of COVAXIN [192]. In the interview he stated that there was little or no transparency from the COVAXIN manufacturers in its trials and approval processes.
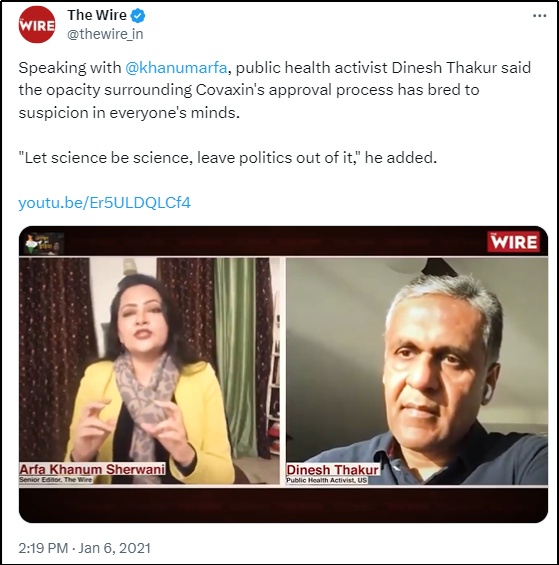
On April 2, 2022 WHO confirmed the suspension for supply of Covaxin (Bharat Biotech) through UN procurement agencies. The suspension was in response to the outcome of a WHO inspection between March 14-22, 2022, and Bharat Biotech has committed to addressing the GMP deficiencies [193]. WHO clearly mentioned that the data, available to WHO, indicate the vaccine is effective and no safety concerns exist. Referring to the article, Thakur wrote an article accusing India’s drug regulators for risking lives while it was a temporary suspension for export only [194]. Later, on June 10, 2022 WHO Emergency Use Listing Procedure (EUL) also evaluated the quality of manufacturing along with safety and efficacy along with WHO Strategic Advisory Group of Experts on Immunization (SAGE) and issued interim policy recommendations for the use of the Bharat Biotech BBV152 COVAXIN vaccine [195]. There was no further follow up clarification given by Thakur on this matter.
While at the time the agenda and propaganda were not clear, in light of the information about the US vaccines and their processes and side effects, which are worse than Covaxin, now it is clear why the campaign was orchestrated. Nonetheless, the damage back then was not in small measures, creating vaccine hesitancy that could have cost lives. Not one of the outlets have clarified/ provided any follow-up stories, because the propaganda is served.
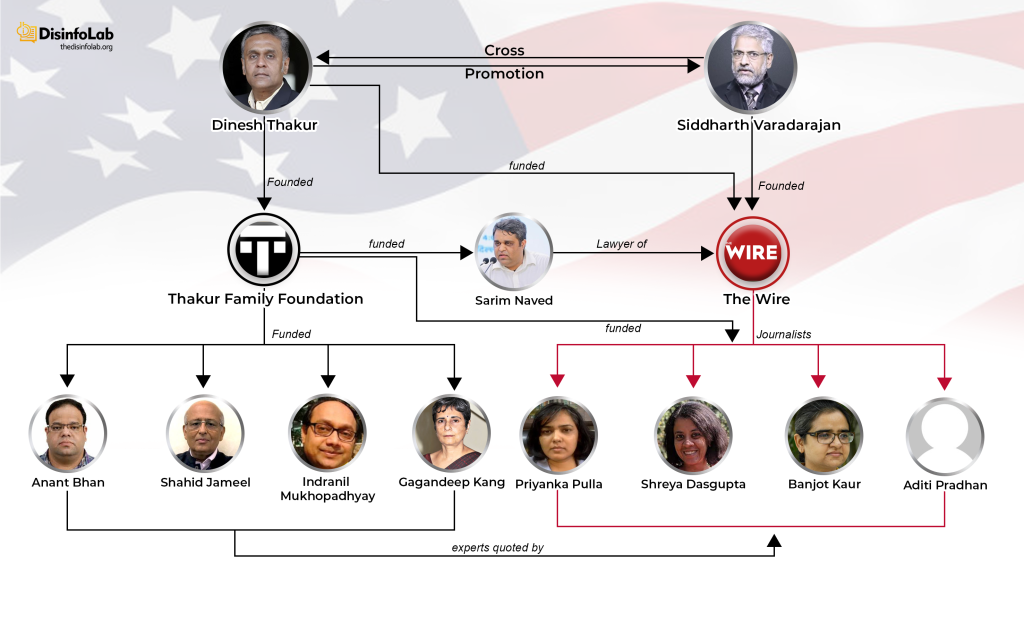
TFF funding meets The Wire’s vision
The Wire did not receive direct funding from the Thakur Family Foundation till 2022. However, the journalists who have received the most funding from the Foundation or have written the most articles during COVID have received a platform on The Wire.
The following word cloud further depicts the theme of The Wire’s articles covered during COVID-19 phase in India between 2020-21.

To further corroborate, here is a sample of a number of The Wire articles critical of the India’s handling of the pandemic between 2020-21.
Priyanka Pulla & Propaganda: a Case Study
During the covid years (2020-21), the Thakur Family Foundation funded several journalists covering Covid-19, including Bangalore-based freelance journalist Priyanka Pulla, a prolific contributor to The Wire, received significant funding. Other The Wire journalists like Banjot Kaur and Shreya Dasgupta also received funding, with their published articles reflecting themes similar to Pulla’s.
Priyanka previously worked at The Outlook Group as a correspondent (from Dec 2007 to 2008). She then joined The Mint as a staff writer for 1.5 years between January 2010 and May 2011. She also worked for The Hindu for six months in 2017 before turning a full-time freelancer in 2019. That is when she was picked by TFF to work on their payroll [196].
Most of Priyanka’s work funded by TFF between 2019-2021 has been featured on The Wire Science. She also wrote TFF-sponsored articles for Livemint, Science.org, and The Quint. Her articles focused on a vast array of topics around COVID-19 and Indian generic drugs. Her articles primarily focused on criticism of the drugs approved by the Indian government during the pandemic and India’s drug testing practice. Topics, Thakur had been crusading against all along [197].
Between 2019-21 Pulla received USD 30,299 from TFF for her reporting on COVID-19 wave in India. In return, she has written a litany of articles criticizing India’s handling of Covid-19 and scepticism regarding the indigenous vaccines.
The Following Word Cloud is the illustration of Dinesh Thakur’s activities on ‘X’ including his interactions with people and topics he spoke about. Not only Thakur sponsored articles quoted Thakur-affiliated experts, he was so pleased with the outcome that his Twitter/X word cloud screams Priyanka Pulla’s name, followed by Anant Bhan, who is the ex-Advisor of TFF and India’s CDSCO which both Thakur and Prashant Reddy spoke about on social media numerous times.
Bloomberg and Priyanka Pulla
It would come as no surprise then that Bloomberg has been spearheading the pharma campaign. Not only it conducted a rather ‘global testing’ of Indian medicine through Valisure, it has onboarded Pulla to write articles on India pharma. And she doesn’t surprise.
TFF and The Wire Journalists
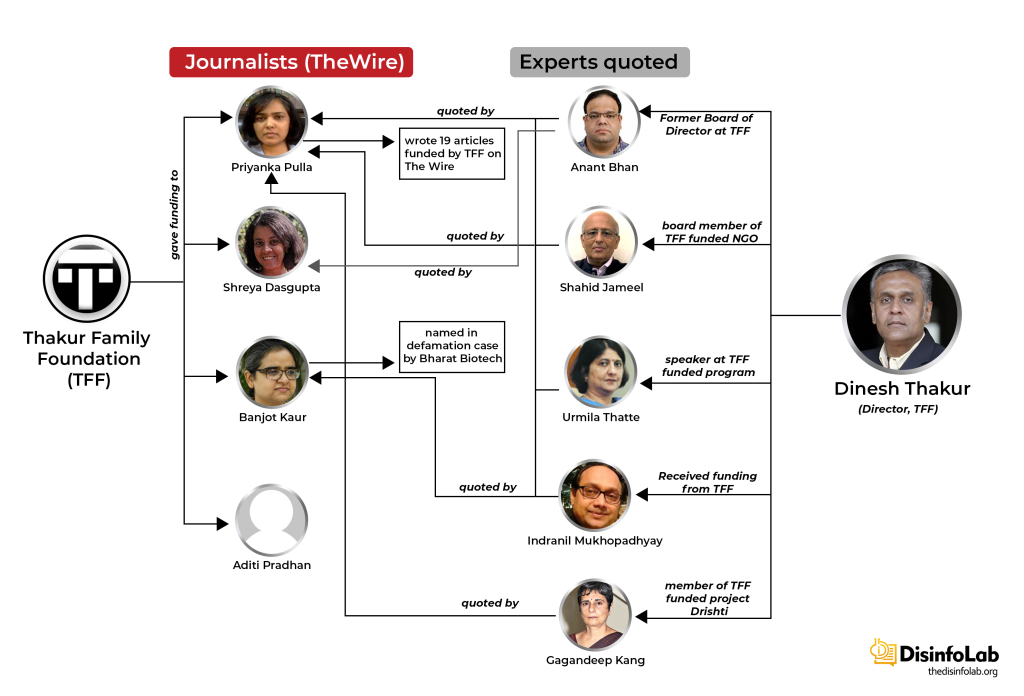
The Loyalty Bonus- The Wire Gets Contract From TFF
The Wire’ did a satisfactory job after receiving handsome amount of funds from Thakur Family Foundation. And while all this time, The Wire’s journalists including Priyanka Pulla, Banjot Kaur, Shreya Dasgupta got grants from TFF between 2019-21, in February 2023, the portal was directly engaged by TFF. According to TFF website, ‘Foundation of Independent Journalism’ also known as The Wire, was awarded to publish a nine-video series on the pillars of public health in India through the lens of national response to the COVID-19 pandemic.

So far, The Wire has published two videos under the series ‘Sehat Sawal Ka’ with the most recent video published on January 17, 2023. [198] [199]
Chapter 13: Nurturing an Eco-System Grants to ‘human rights activists’
A common feature of all anti-India narrative actors is that they invariably turn to each other to further their propaganda (and making our job easier). Thakur Family Foundation (TFF) has also sanctioned grants to a New York-based lawyer and author Suchitra Vijayan under the Civil Liberties category. For the unversed, Suchitra Vijayan is the co-founder of the Polis Project which was incorporated in 2016.
Suchitra has been active on Kashmir issue since 2016. She was also an attendee at the 12th International Peace Conference on Kashmir in 2015 organized by the convicted ISI mole Ghulam Nabi Fai [200]. Besides, she has also made appearances on the Pakistan-sponsored Stand with Kashmir (SWK) platform [201] [202] [203]. Amid that, Suchitra and her Polis Project were being groomed as a major anti-India platform before its untimely dissection by Disinfolab and reported its Pakistani connect.
Thakur Family Foundation granted her USD 10K in 2021. Suchitra has several articles on Kashmir and human rights issues in India. The grant was made to The Polis Project, Inc. to study judicial and administrative accountability in cases of custodial death in India [204].

However, upon cross-checking the website of Polis Project for their coverage of the given topic they received grants, we found a five-report series under the series [205]. Two reports of the series were published in 2021, on July 2 [206] and July 11 [207] respectively. The next three instalments were published in 2023 on July 21 [208], July 24 [209], and July 27 [210] respectively. Notably, none of them mentioned the disclaimer that the series was funded by the Thakur Family Foundation.
As to what end such sponsored narrative is: on April 26, 2021, Suchitra also wrote an article titled ‘India Desperately Needs Biden’s Help to Address the Covid-19 Surge’ as the US announced shipping US-manufactured vaccines to help other countries [211]. On that day, Suchitra was a health expert.
The Long Game
We noticed that Thakur has been ‘diversifying’ his portfolio. Other than funding to media for stories on health and medicine, he has also been branching out to other domains, most notably to civil society groups. His funding to civil groups have been increasing over the years.
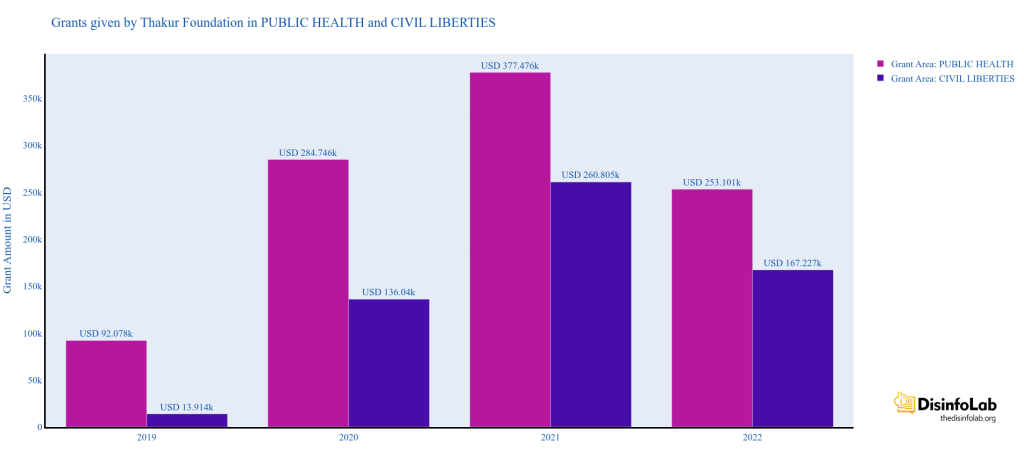
Worth noting that starting as ‘health activist’, Thakur also got ‘interested’ in other issues in India. There could be multiple reasons for it, including the fact that the machinery that runs Thakur types has an existing eco-system that can help him and in turn get help from him. Over the years, GoI has restricted foreign funding for multiple institutions. In 2025-16 alone it was to the tune of 10k Cr. Hence, the deprived organizations would need the funding, and Thakur Foundation could be a good source.
Thakur’s operation also has a potential to generate its own income, making it self-sustainable. By anointing himself as the arbiter of ‘good vs bad medicine’, Thakur can command a premium from Indian pharma industry. By widening his reach and brand through ‘rights’ organizations and civil society groups, Thakur could add to his worth.
Moneylife Foundation & TFF Funding
In September 2019, Dinesh Thakur attended an event titled, Why India Can’t Produce Best Quality Generic Drugs, Asks Dinesh Thakur, the Ranbaxy Whistleblower [212]. The event was organized in Mumbai by Moneylife Foundation.

Moneylife Foundation (MLF) is a non-profit organization dedicated to financial literacy, consumer awareness, and advocacy in India. It was co-founded by financial journalist Sucheta Dalal and her husband and Chartered Accountant (CA) Debashis Basu [213]. Following exposing the Harshad Mehta scam, Debashish Basu and his wife Sucheta Dalal established Moneylife Foundation (MLF) in 2010. MLF content focuses on financial analysis and activism, catering to a niche audience interested in finance and consumer rights. MLF received grants from Thakur Family Foundation for different periods between 2019-21. In 2019, TFF granted USD 27,584 for supporting the organization’s charitable causes [214].
RTI Centre Funded by TFF
MLF launched its Right To Information (RTI) centre in 2017 [215]. As per its website, its RTI centre was launched with ‘generous’ support from Dinesh Thakur from the US. As for now, MLF lists Thakur Family Foundation as one of its patrons. But it is worth noting that at the time MLF started its RTI, from the ‘generous’ help from Dinesh Thakur, he was residing in the US and he hadn’t started Thakur Family Foundation (TFF) yet [216]. While it is not known how much money MLF received from Thakur to start its RTI centre, as per financial filings of TFF, In MLF has received USD 27,684 (2019), USD 45,526 (2020), and USD 22,907 (2021) in total to date, barring the amount it received in 2017.
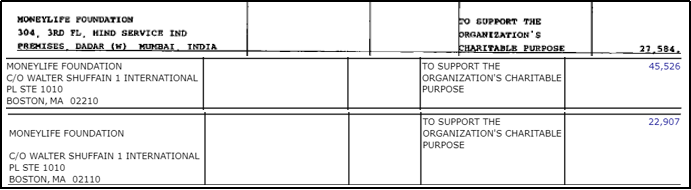
Worth also noting that Thakur being a US citizen and OCI holder, was not eligible for RTI. Hence, it was a dubious means to access what was not accessible directly. And what did the RTI do?
Campaign against Sun Pharma
In November 2018, MLF published a story that it got hands on a 150-pager whistleblower complaint against Sun Pharma and its promoters Dilip Sanghvi (Founder) and his brother-in-law Sudhir Valia filed to Securities and Exchange Board of India (SEBI) in mid-September. Following that incident, Sun Pharma’s stock price plummeted by 40% in the stock market. It was Sun Pharma that had acquired The Ranbaxy in 2015 [217].
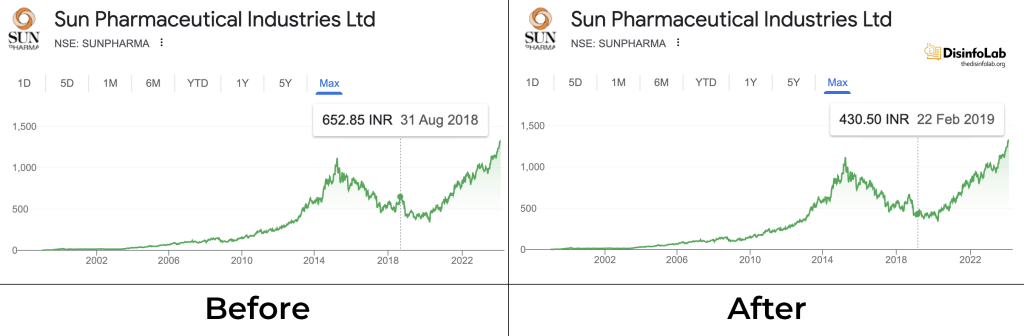
Chapter 14: Citizens for Affordable, Safe, & Effective Medicine
While setting the tone to getting the literature published via funding several portals and organizations, and journalists in India, Thakur also parallelly started another group named- Citizens for Affordable, Safe and Effective Medicine (CASEM) in May 2020, at the time of the peak COVID-19. The website of CASEM was registered on May 9, 2020 [218].
As per its website, CASEM is a collective of like-minded individuals who seek ‘Make in India’ medicine more affordable, safe, and effective for patients [219] [220]. But the “like-minded individuals” consist only of Dinesh Thakur himself. This company shares the same address as Dinesh Thakur’s The Thakur Foundation, but an important point to note is that this company is not registered anywhere.


Thakur has been using the CASEM website for filing his petitions regarding the Indian pharma industry to the Indian health ministries and departments. While filing the petitions, Thakur uses his own manufactured resources, either reports authored in his name or the funded by him. For example, he had filed a petition dated October 24, 2021, regarding the Committee to draft a new Drugs, Cosmetics and Medical Devices Act [221].
He has referred to an article of Live Mint by Priyanka Pulla, who has been working on grants by TFF since 2019, to highlight how “weak drug laws in India are taking lives”. Another source quoted by him in the same article is Shreya Dasgupta, another beneficiary of the Thakur Foundation, criticizing the Ethics Committee and the Indian Council of Medical Research (ICMR). He has referred to multiple such reports by Shreya Dasgupta funded by his Foundation.
Parallelly, he has also written blogs on the filed petitions, again referencing his articles. In instances where the references do not seem to be funded by him, it turns out that those articles are based on a other reports/ articles curated/ funded by Thakur. For instance, in his CASEM’s blog on petition proposing a new roadmap for India’s new drug regulatory law, he has referred to an article by IndiaSpend to highlight flaws in India’s drug regulatory system, which in turn is based on Thakur’s report [222] [223] [224]. So far, via CASEM, Thakur has filed around eight petitions [226].


In other words, Thakur has deployed a circular deception: he files petition/ advocacy using articles funded by him, which in turn are amplified individuals funded by him. These petitions are used as references to generate more articles, and all of this to create a negative impression about pharma industry.
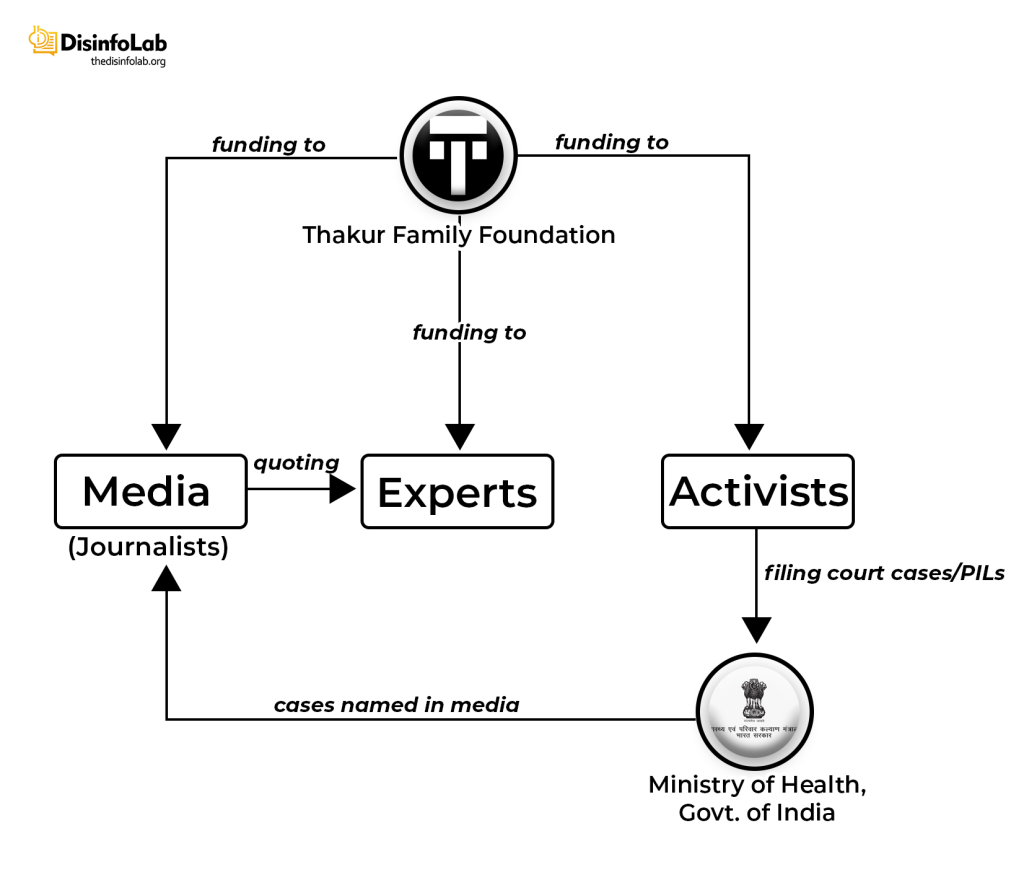
Narrative Wars Don’t Come Alone
In 2020, the Thakur Family Foundation funded two stories on topics about Kashmir. TFF engaged a New Delhi-based Swagata Yadavar, who has worked with The Print, IndiaSpend, and The Week. She has also written on various portals including Cancer World, Al Jazeera, Business Insider, Article 14, and Scroll among others.
Swagata was granted USD 4,126 for writing two articles which were published on Scroll and Al Jazeera. She wrote two articles on Kashmir including on the situation in J&K during the COVID-19 lockdown and the ‘harassment’ of Kashmir journalists on the international portal Al Jazeera.
The articles were co-authored by a Srinagar-based journalist Ather Parvaiz who writes for various portals including Down to Earth, The Print, The Wire, The Third Pole, Scroll, and Al Jazeera among others. As per his bio on Eurasiareview.com, he is described as a writer based in ‘Indian-administered Kashmir’.
On October 28, 2022. Athar Parvaiz wrote an article titled ‘After Gambian tragedy, Indonesia bans cough syrups’ referring to the ban on cough syrups by the Indonesian government due to Acute Kidney Infection (AKI). The reporting of the Indonesia case was done in such a way, with headlines spun that it would seem that Indian syrups were responsible for deaths in Indonesia just like for the deaths of children in Gambia. In contrary to the fact that the cause of the tragic deaths could be linked to by several reasons, as accounted in Chapter 1. The article also promoted Thakur’s book, The Truth Pill.
Thakur echoes the narratives on Kashmir against the interests of India on numerous occasions. There have been occasions when Thakur promoted such tweets on Kashmir by quoting/ reposting tweets from Ashok Swain and Aakar Patel but the latter would later delete their tweets.
Part IV: The Ultimate Target – the ‘Indian Way’
Chapter 15: Indian Vaccines at Target India Embarks on a Forbidden Path
India Embarks on a Forbidden Path
While India’s brand as pharmacy of the world was bad enough, it was tolerable, as the branding stayed around generic drug, a thing for poor people. However, India’s attempt to enter into the elite club was difficult to digest for the gatekeeps of the club – the US pharma lobby. It was akin to India’s foray into space. A low cost but effective solution. While to everyone it may seem like a clean and desirable solution, one would not get the impression if one reads the opinion of Western elites and western media. The message had to be delivered, and delivered hard.
The sporadic attempts at Indian pharma had continued even before Covid. But coinciding with vaccine development, it had exploded. India had been sending medicine to hundreds of countries over decades. But suddenly, there has been an explosion in reports about ‘bad medicine’ from India. It did not matter whether Indian medicine was even involved or not, the narrative had to be established.
But nothing had been comparable to what we witnessed during the process of India’s attempt for a vaccine. A coordinated alarm was sounded from various corners at each stage of vaccine developments. The beneficiaries were obvious- the Western pharma. And some more enthusiastic advocates actually called for India to halt its vaccine programme and import the US vaccines. It is not clear how many of them were aware about the process the US manufacturers were adopting, because they were not significantly different from India. Moreover, unlike Indian vaccine which went on a tried and tested method, some of the US vaccines attempted a technology (mRNA), which is not yet fully evaluated for its side effects and long term effects.
It was quite a coincidence that many of the votaries of such US vaccines (Pfizer/ Moderna etc) were also associated with Thakur. In fact, since the beginning of the talks of indigenous vaccines, Dinesh Thakur had been quite critical of their quality and the entire process and evets pertaining to it as reflected from the articles written by himself on his own website or on other websites as well as the articles funded by him.
Upon closer inspection, most of these articles and reports were found to be either from TFF funded media portals or TFF funded journalists, as noted in the preceding sections. Articles and opinion pieces appeared from these portals, questioning the efficacy and safety of Indian vaccines, often citing the lack of published data or expressing reservations about the expedited approval process.
Several instances of this section of Indian media reflected a somewhat sensationalist approach, emphasizing uncertainties and potential drawbacks. At the fore front of this onslaught on the indigenous vaccines was The Wire. The reportage of the Bharat Biotech’s vaccine was so overboard that it led to The Wire getting slapped with a suit of 100 crores and was instructed to take down its 14 articles allegedly defaming Bharat Biotech.

In addition, some of the others who vouched for/ promoted foreign vaccines were also somehow connected with the Thakur nexus:
- Dr. Sumaiya Shaikh [226]
- Kunal Rajnikant Purohit [227]
- Priyanka Pulla [228] [229]
- Dr. Cyriac Abby Philips (@theliverdr) [230]
- Rohini Singh [231]
Defamation Suit Against The Wire
The shrill from the Wire against Bharat Biotech was so high that it eventually attracted reaction. On February 23, 2022, Bharat Biotech filed a Defamation Lawsuit against The Wire for INR 100 Cr, and also demanding the takedown of 14 articles that allegedly defamed BB [232]. The Court upheld the contention, and ordered The Wire to take down those articles, to which The Wire has complied.
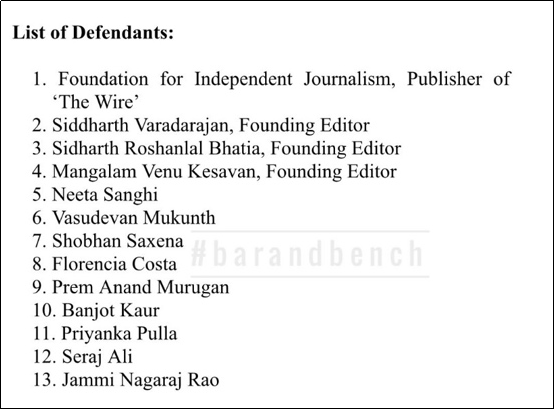
Chapter 16: The Level of Propaganda: Aparna Gopalan a Case Study
During Covid-19 period, Aparna Gopalan, news editor at Jewish Currents [233] and a doctoral candidate in Social Anthropology at Harvard University published an article [234] “India’s Vaccine Makers Are Pandemic Profiteers, not Humanitarians” targeting Serum Institute and Bharat Biotech. The article suggests that India’s vaccine makers were engaged in profiteering by selling vaccines at high prices to private hospitals, resulting in substantial profits. The comparison with other vaccine producers, such as Pfizer and Moderna, implies that Serum Institute’s profit margins were disproportionately higher. It seems in order to paddle her narrative she totally forgot to do overall cost comparison of all said vaccines as Covaxin and Covishield were way cheaper for Indian citizens.
| Vaccine | Price |
| Bharat Biotech’s Covaxin | For govt: Rs 600/dose to statesPrivate hospitals- Rs 1200/dose For citizens: Free at government centresRs 250 in private hospitals |
| AstraZeneca – Serum Institute’s Covishield | For govt: Rs 300/dose to statesPrivate hospitals- Rs 600/dose For citizens: Free at government CentresRs 250 in private hospitals |
| Pfizer’s vaccine | $19.50/dose (Rs 1,431) to states |
| Moderna’s vaccine | $32-$37/dose (Rs 2,348-Rs 2,715) to states |
influencers and activist. There were so many red flags in the new mRNA vaccines, but it didn’t raise any scrutiny from our ever-vigilant journalist, who had declared Indian vaccines sub-par to begin with.
Chapter 17: Indian Vaccines vs Foreign Vaccines
It is challenging to compare vaccines head-to-head due to differences in study designs. However, some of the major steps are common. The SOPs of vaccine trials consist of 3 standard phases: research and discovery phases; preclinical trials; and clinical trials. The last phase, itself consists of 3 sub-phases in which the number of volunteers increases from 10- 1000s [237].
Concerned over Clinical trials of the Vaccine
Indian vaccine process was criticized for hasty trials, issues with clinical trials, not sufficient trials and time etc. And these were the reasons why many eminent personalities demanded western medicine. What they did not know and did not care to check was that their article of faith was grossly misplaced. The problems with their trusted Western vaccines were way more than they were worried about.
Poorly regulated testing system in Africa, Asia, Eastern Europe, and Latin America that is dominated by private interests and that far too often betrays its promises to patients and consumers [238].
Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal COVID-19 vaccine trial raise questions about data integrity and regulatory oversight, as reported by Paul D Thacker in BMJ.
An unproven technology
Both Moderns and Pfizer deployed mRNA technique, which remains an unstable technology with the fear that it might interfere with the human genome. The companies have not come clean about the claims, and several contradictory claims have been forwarded, including a claim that COVID-19 mRNA vaccines contain DNA contaminants is based on a study of vials of “unknown provenance”; no evidence COVID-19 mRNA vaccines can alter DNA in people [239]. However, later the claims were modified, stating that COVID mRNA shots “may have the ability to alter the human genome”. But our Indian well-wishers were happy with an unproven technology compared to a proven one, used by Indian company.
The Hasty Approvals
As shown in the COVID-19 vaccine approval comparison, the timeline followed by all the vaccine makers were more or less similar. BUT the Indian vaccine makers and medical authorities face barrage of accusations for ‘hasty’ approval. The same time frame of Western ones was proof of efficiency.
Preliminary tests conducted by Pfizer in June 2022 were done on eight mice. The actual number of mice involved in the recent trials remains unclear. The boosters made by Pfizer-BioNTech and Moderna were approved by the FDA on Mon (Sept 10, 2023) [240] [241]. On Tue, a panel of advisers to the CDC voted 13-1 in favor of its broad use for individuals six months and older due to fears of increased respiratory illnesses this fall and winter [242] [243].
The Covid PCR protocol paper, co-authored by Marion Koopmans, Netherlands based virologist, was bypassed peer-review and was published within one day. It was then declared the gold standard by a WHO committee the very next day, a committee on which Koopmans was a member.
Side Effects, reported so far
The advocates of Pfizer/ Moderna vaccines may or may not know, but there has been a cascade of reports about the possible side effects of these vaccines. Not to mention the cost. And one of the reasons the Govt claimed for not allowing these vaccines in India was that they demanded immunity from prosecution. Now it is becoming clearer why, seeing their side and long-term effects.
The most important and most common complications are transverse myelitis (more about Pfizer, Moderna, AstraZeneca, and J&J), Bell’s palsy (more about Pfizer, Moderna, AstraZeneca), GBS (more about Pfizer, AstraZeneca, and J&J), and the first manifestation of MS (more about Pfizer), concluded by Hosseini & Askari [244] [245].
Several individuals develop inflammatory cardiomyopathy, thrombosis, neurologic problems, and other rare conditions after COVID-19 vaccination, especially based on nucleic acid. In silco reports indicate myocardial inflammation in individuals with vaccine-related myocarditis and lymphocyte infiltrate, suggesting an autoimmune-like attack [246].
In 2023, 158,000 more Americans died than expected. That’s more than all wars combined since Vietnam.” While the FDA blames this on smoking and bad diet, Dr. Pierre Kory has another explanation: vaccines. Deaths among 35–44-year-olds rose 26% this spring, while deaths among 25-34-year-olds rose 19% above pre-COVID levels.
In November 2023, Texas Attorney General Ken Paxton filed a lawsuit against Pfizer alleging that Pfizer made false statements about the effectiveness of its Covid-19 vaccine and tried to stop people from talking publicly about its problems [247].
“Pfizer engaged in false, deceptive, and misleading acts and practices by making unsupported claims regarding the company’s Covid-19 vaccine, in violation of the Texas Deceptive Trade Practices Act,” Paxton said in a statement,
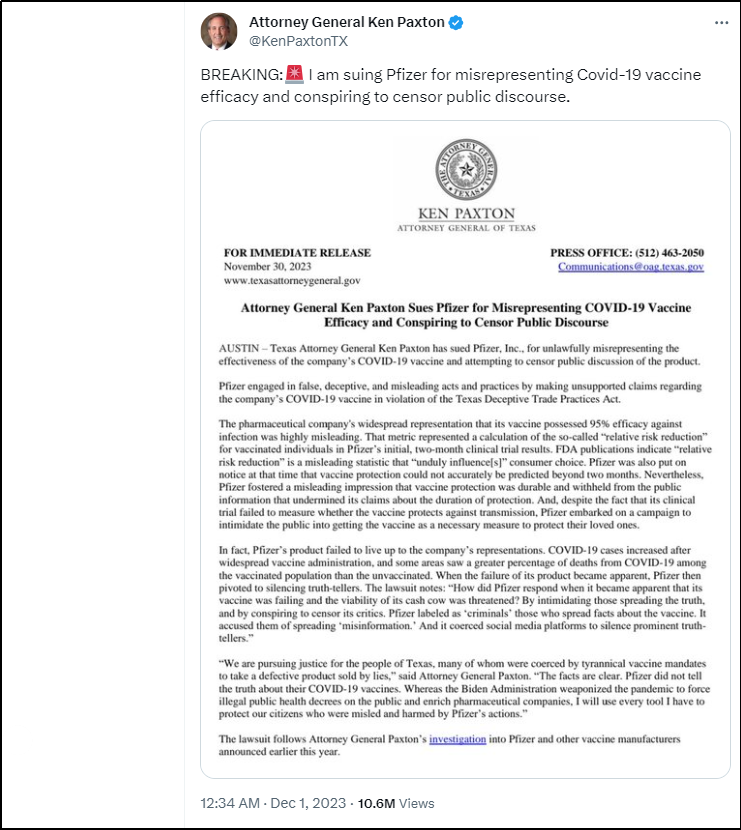
There have been multiple reports about the ‘sudden increase’ in deaths from diseases after the vaccines were introduced, and many a diseases have re-surfaced which had become almost non-existent. The most devastating affect has been in the increase of sudden heart attacks, most notably in the young and otherwise healthy individuals.
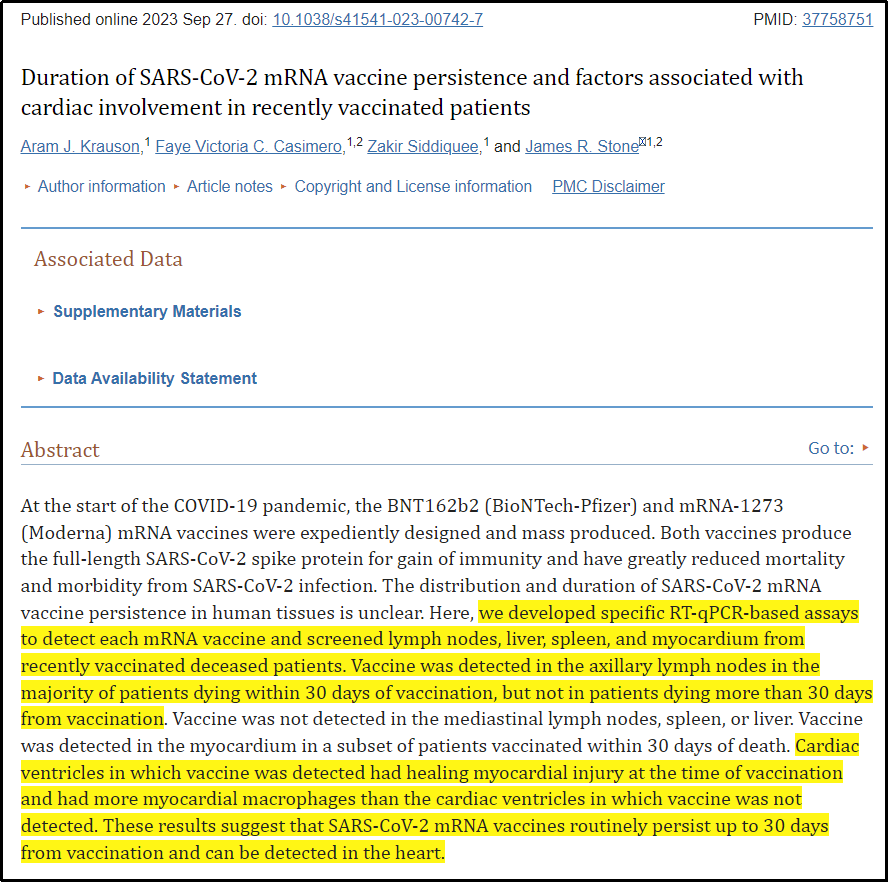
So if the technology was unproven, the cost was high, the foreign vaccine were not willing to share responsibility and the process time was same, why indeed so many called for those vaccines.
Big Pharma and Big Money
Big Pharma & FDA: Biopharmaceutical Industry Provides 75% of the FDA budget. Not to mention the revolving door between Pharma and FDA. It raises concerns regarding the FDA’s objectivity in drug approvals, suggesting potential industry influence due to the pharmaceutical sector [249].
Big Pharma & Govt: White House adviser Anita Dunn’s firm works closely with Pfizer [250]; Biden’s domestic policy adviser Susan Rice holds $5 million in Johnson & Johnson shares [251]; White House science adviser Eric Lander holds $1 million in shares of BioNTech [252], co-developed Pfizer’s vaccine.” [253]
Big Pharma Money to Senators: Senate Finance Committee members received hundreds and thousands of dollars as political contributions from pharmaceutical PACs to both Republican and Democratic committees, and also individual contributions from CEOs to senators on the finance committee. The total contribution amounted to $1.6 million to the campaign committees of 27 out of 28 members of the Finance Committee [254] from seven big pharma companies- AbbVie, Astra Zeneca, Bristol-Myers Squibb , Johnson & Johnson , Merck, Pfizer and Sanofi
Out of 97 US lawmakers, listed by NYT in 2022, 26 have invested in the pharma sector, which includes Pfizer (2) and J&J (2) with conflict of interests at 308 points [255].
A STAT analysis of thousands of pages of congressional disclosure forms found that about 30% of senators and 20% of representatives held assets in biomedical and healthcare companies, mostly in Pfizer, Johnson & Johnson, Merck, and Abbott Laboratories [256]
Senators who received at least $100,000 from the pharma PACs include (as accessed by the Forbes):
- Mike Crapo (R-Idaho) – $119,000
- Rob Portman (R-Ohio) – $113,000
- Johnny Isakson (R-Georgia) – $107,000
- Tim Scott (R-South Carolina) – $101,000
- Tom Carper (D-Delaware) – $100,000
Thakur Family Foundation
The funding in India through one just front – TFF – alone has been INR 14-15 Crores for four years. There would be multiple fronts. And Indian journalist and influencers come very cheap. However, they should thank their stars that these vaccines were not allowed in India. They are being sued for misrepresentation and fake claims, and the even bigger worries are the side effects.
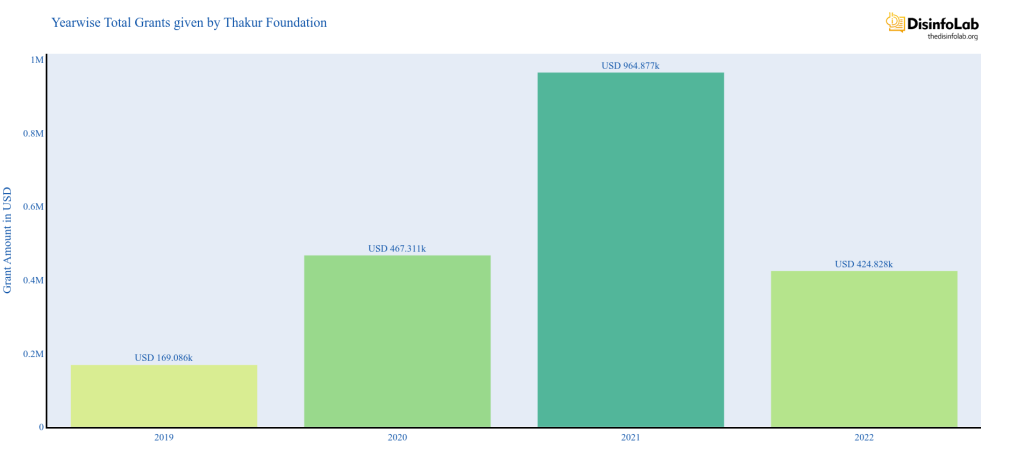
Chapter 18: India’s Covid Story: a suitable Deflection
India’s vaccine journey was not the only eye-sore. Covid had dealt a mortal blow to a Hollywood crafted fairy-tail – the invincible USA. The ‘American exceptionalism’, built through the work of fiction got shredded with the first reality check of modern times. What was worse, some of the third world developing countries were doing better. Far better. The story needed to change, and one of the most suitable targets was India. There could be many reasons for this attention: given India’s size, it was obvious there would be problems, and visible ones. The numbers would look astonishing in any context. The country was freely accessible, despite the repeated chant of lack of freedom. Most of all, China was doing far better, and was inaccessible. Russia was small in terms of population, and inaccessible. Other countries of note were, unfortunately, White. Hence, India.
It was applied at every stage. Suddenly, comparisons were being made with small and developed societies like South Korea and Taiwan in terms of testing. Accessibility of hospital and oxygen were obviously going to be a problem. No society could have prepared so quick for such a number. Not even the US. But the most effective numbers were death numbers. All media used gross numbers to compare Indian death tally, because per capita, the West was looking ugly.
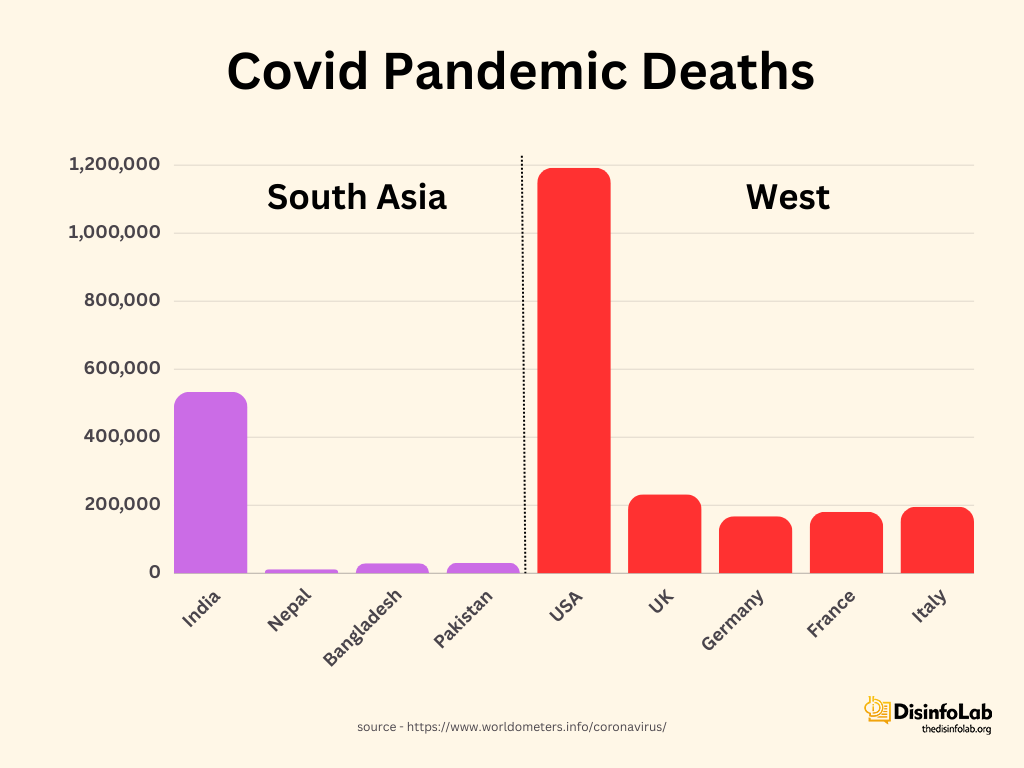
The trope used was: India was under reporting its death numbers. However, if one were to look at the causality data of entire South Asia, which share almost similar genetic profile, climate and living standards, the death rate was in similar range. If anything, Indian death numbers were slightly higher. On the other hand, the Western world also had similarities in the range of causalities. BUT India was under-reporting. And by how much? Media reports claimed that India was under-reporting by as much as 10 times! However, no reasonable or scientific basis was provided about the magic number of 10 times.
But there was: 10 times Indian causality would make it more visible than the US. One wonders, why the generosity.
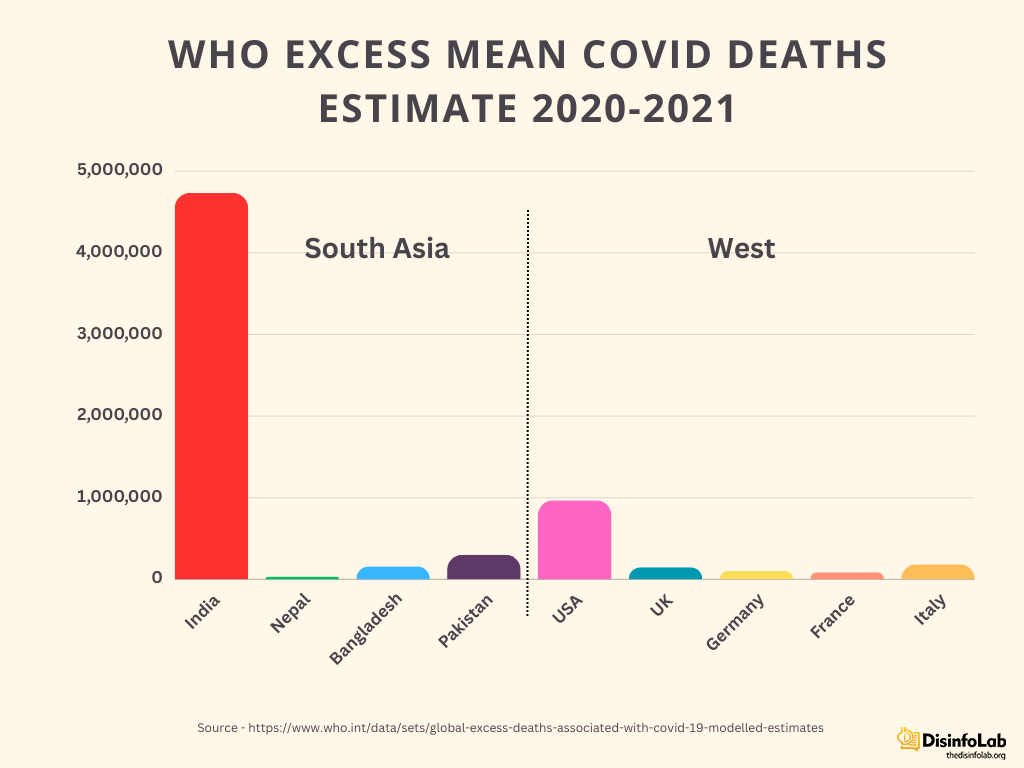
The story did not stop here. If India succeeded in managing Covid, and succeeded in developing vaccine, it would be a major question on the Western liberal order, which believes that other than the White world, democratic systems can’t survive elsewhere. The only exceptions are mostly westernized countries – such as South Korea, Japan, and Taiwan etc. Hence, India has to be dragged even further, and one way to do is to compare with Pakistan. While Pakistan did fare better than many countries in West, primarily by not doing anything, it was rather baffling as to how it could have fared significantly better than India with a poorer health infra and virtually no covid policy. The mandarins in Media had to stretch all reasons to make it appear better, where one claim in one article was diametrically opposite to another claim in another article.
However, at a time where headlines are all that one reads, this was not a problem to be worried about. And like every other lie, this eventually will become a fact, to be quoted in research articles and book.
The Larger Agenda
Other than generic, medicines and Indian vaccines, one more aspect of Indian health system is at Thakur’s target- traditional Indian medicine system.
Thakur engaged in a discussion on Ayurveda alongside Dr. Abby Philips, with a book promotion event [257] [258]. Dr Abby aka the Liver Doctor has been running his own crusade against Ayurveda. In the name of his ‘scientific’ approach, he brings out the ‘allelopathic framework’ to judge the alternative medicine, and declares that traditional medicines are not scientific. It is like bringing the cat as a framework for animal to evaluate dogs, and concluding that dogs are not animals. It is no surprising that other TFF grantees have also devoted their efforts to try discrediting Ayurveda and alternative medicine. All in name of the ‘science’.

It is akin to the chorus that was sung in the West during the peak of Covid – ‘trust the science’, which was used to not only push through unscientific, unproven, and in many cases dangerous policies, but was used as a puritan tool to quash, cancel and silent any questioning or contrary opinion.
The Indian votaries of foren vaccine should thank their stars that their high-pitch propaganda didn’t compel the authorities to give in to their demands, given the side effect the western societies are witnessing. They should thank their lack of credibility as well.
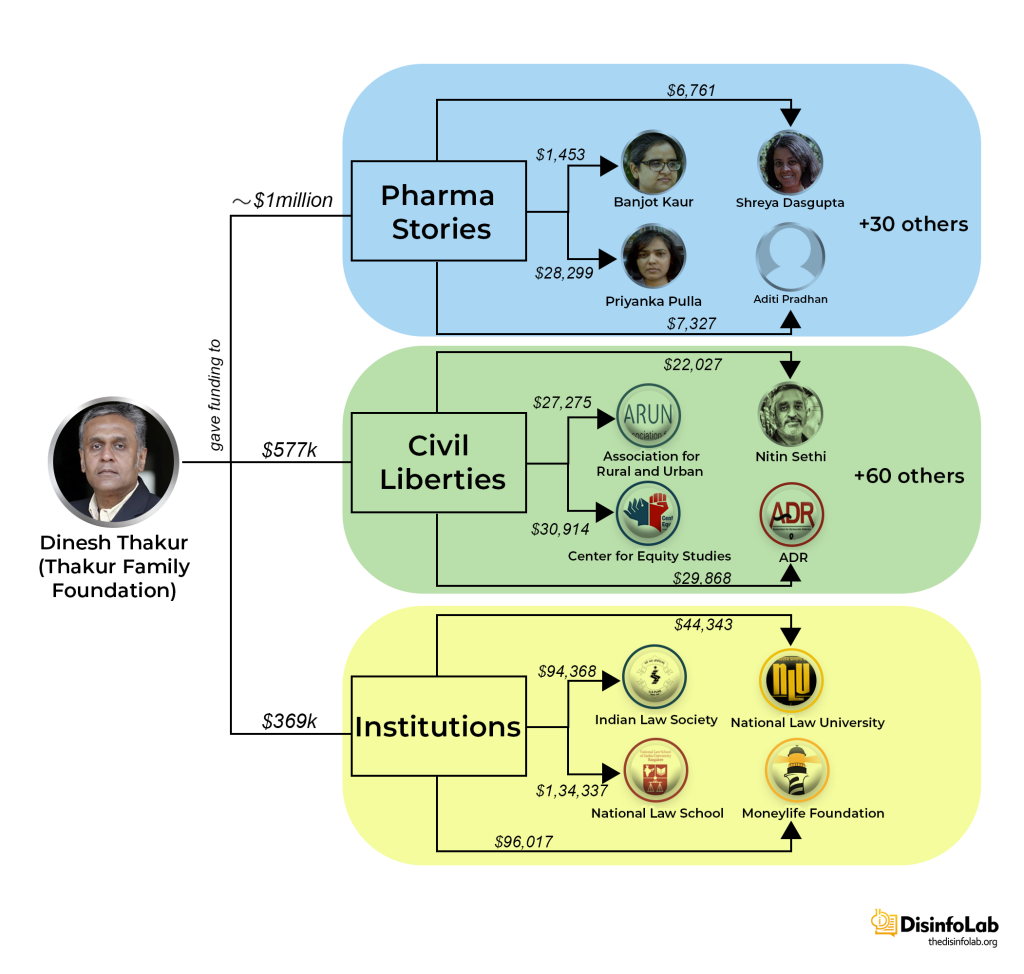
Conclusion
Thakur is merely a pawn, a front for an enterprise that is aimed at multiple targets in layers. At the first layer, it targets individual companies, such as Ranbaxy, which have come in dispute with the US companies. Using this as base, the next layer targets the Indian generic medicine, which in general is a threat to the US big pharma, which sells medicines at multiple times of cost, and has been pushing against generic medicine for a long time, which might eat into their profits. Not surprisingly, Thakur and Co. have devoted considerable resources to target Indian generic medicine in general. At the third layer, this ruse is used to target the overall Indian pharma, thereby systematically denting the image to prevent it from ever becoming a competitor to the US pharma. The fact that such attacks have intensified once India decided to embark upon the indigenous vaccine tells its own tale. The sheer pitch for importing costly and unproven vaccines from abroad was rather obvious for its tone.
However, there is even larger game at play. While the above objective only serves the Western market, India itself is a big market. If India had not developed covid vaccine, it would have added some more billions to the profit for the US pharma. However, to capture the market, the Big Pharma not only has to discredit the Indian pharma but also the Indian traditional medicine system, especially the Ayurveda which commands a significant market portion, and is getting increasingly popular given the new found emphasis to go herbal and natural. Not surpassingly, many such crusaders also happen to be bed-fellows for Thakur.
The most outer layer, and the overarching target of the campaign merges with the larger narrative at play against India – targeting India’s image globally. What else could explain Thakur’s foray in funding known India baiters, such as Suchitra Vijayan, who have been exposed repeatedly for fake news and for colluding with the Pakistani agenda, literally having an admin from Pakistan running the social media of her project Polis. But this is nor new nor first. We have noted that the ‘accidentally dropped’ toolkit by Greta regarding the farmers’ protest called for targeting India’s branding of Yoga and Chai. Earlier, the same cabal was targeting India’s branding of ‘non-violence’ and Mahatma Gandhi. It is the same cabal that provides fodder for boycotting Indian products every now and then.
Tragically, while the string-masters sitting in the developed capitals have very obvious economic and parochial motives, the largest part of this travesty is conducted through people of Indian origin. Apparently, no other society produces as much self-hating and self-loathing individuals as Indian society. It remains beyond comprehension as to what alure these individuals are so eagerly selling out to, which not only hurts India in abstract such as India’s image, but in very concrete terms, its economy, and thereby its people. Al this while, pretending to be some sort of activist and hero on social media.
Whether they ever question themselves about the premises they have come to internalise, even in the face of facts?
Would they?




